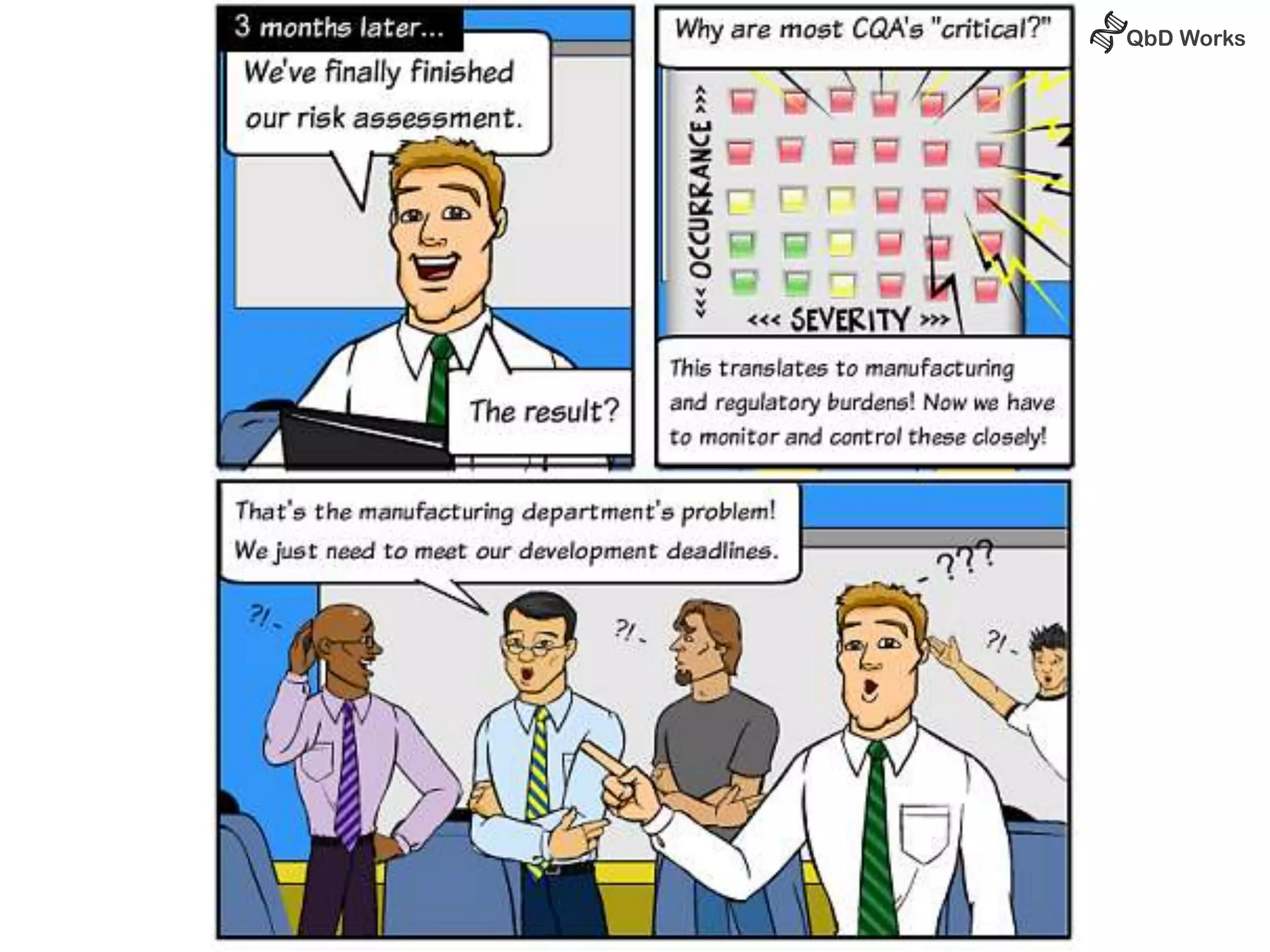
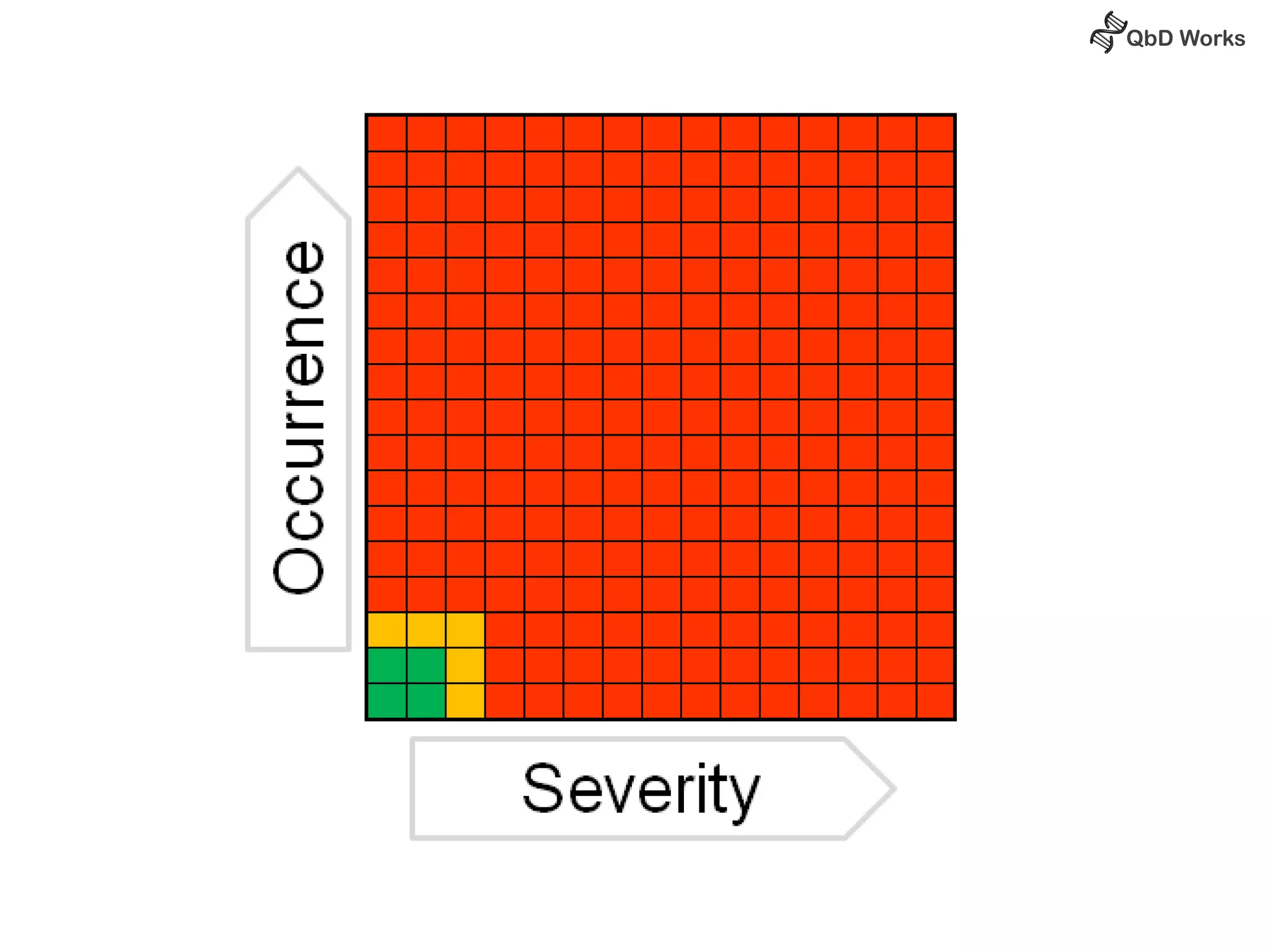
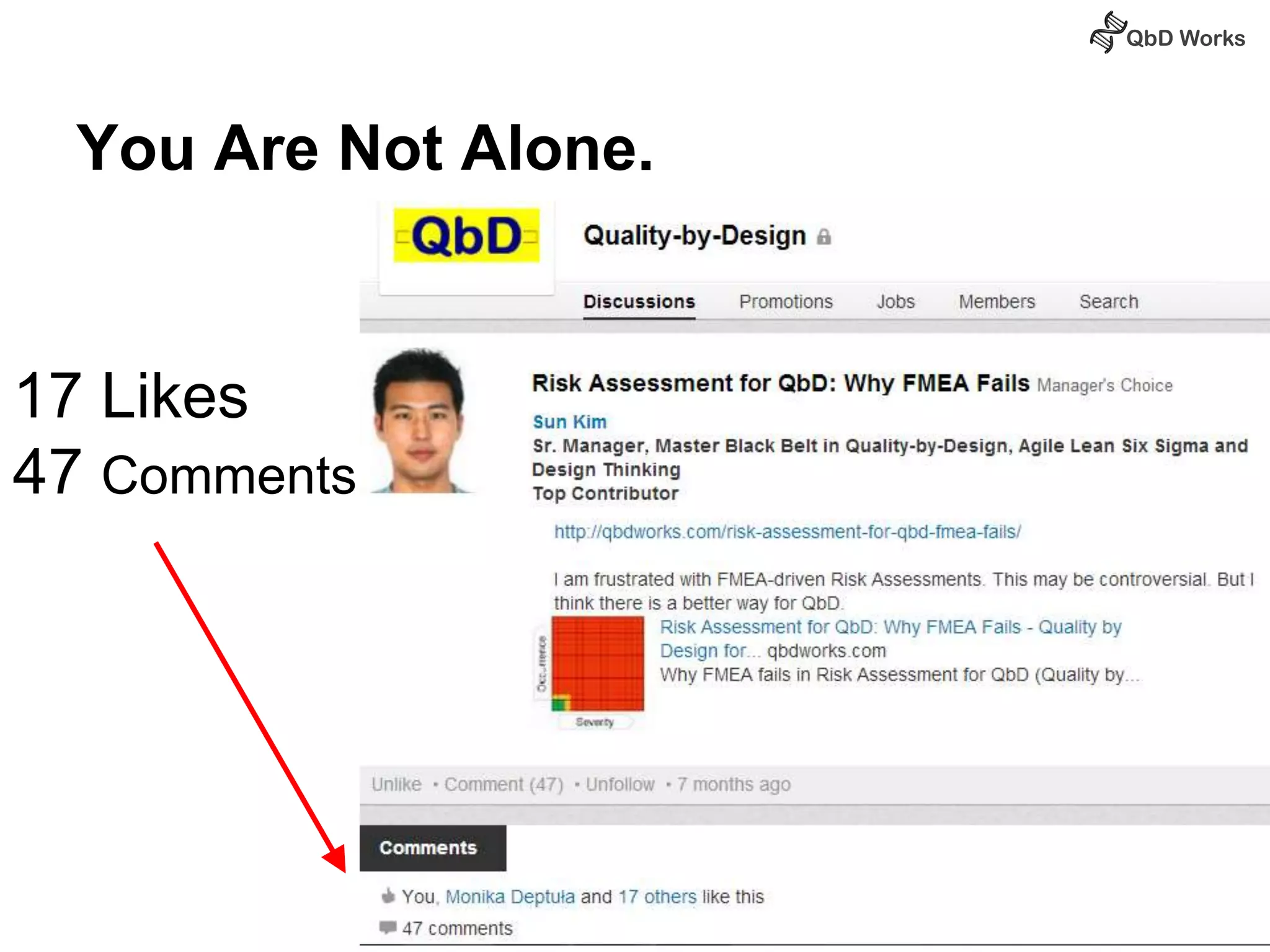
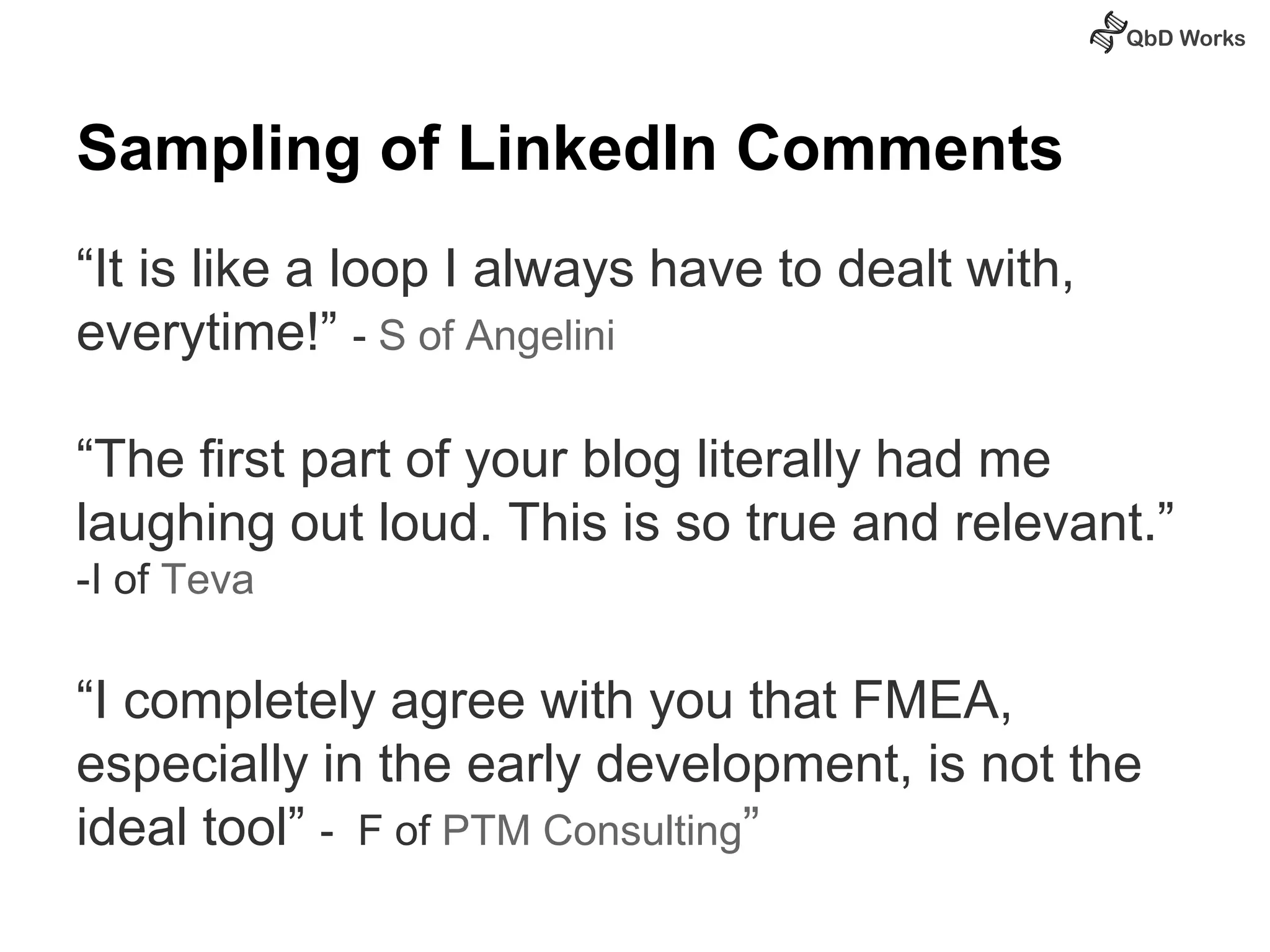
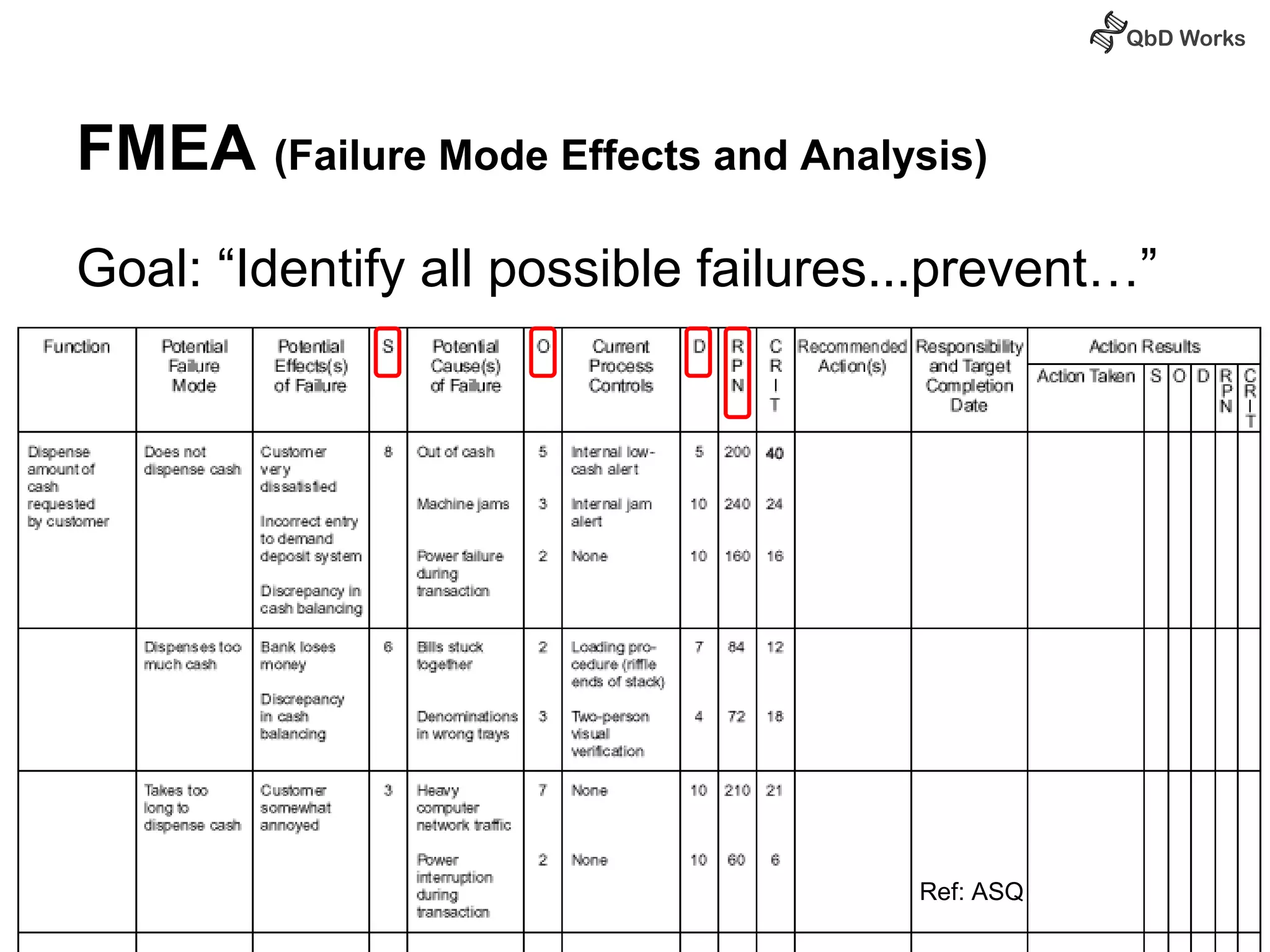
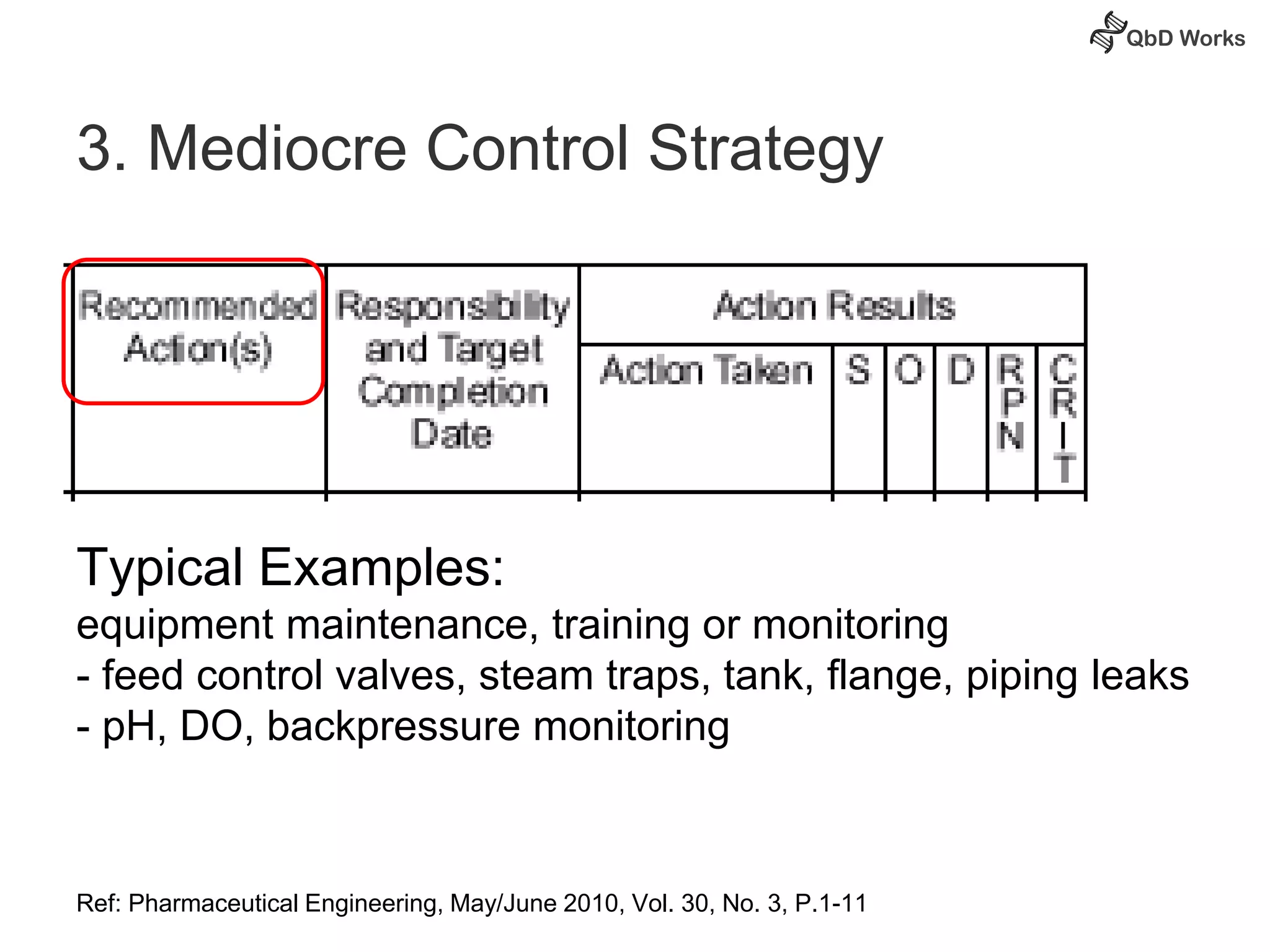
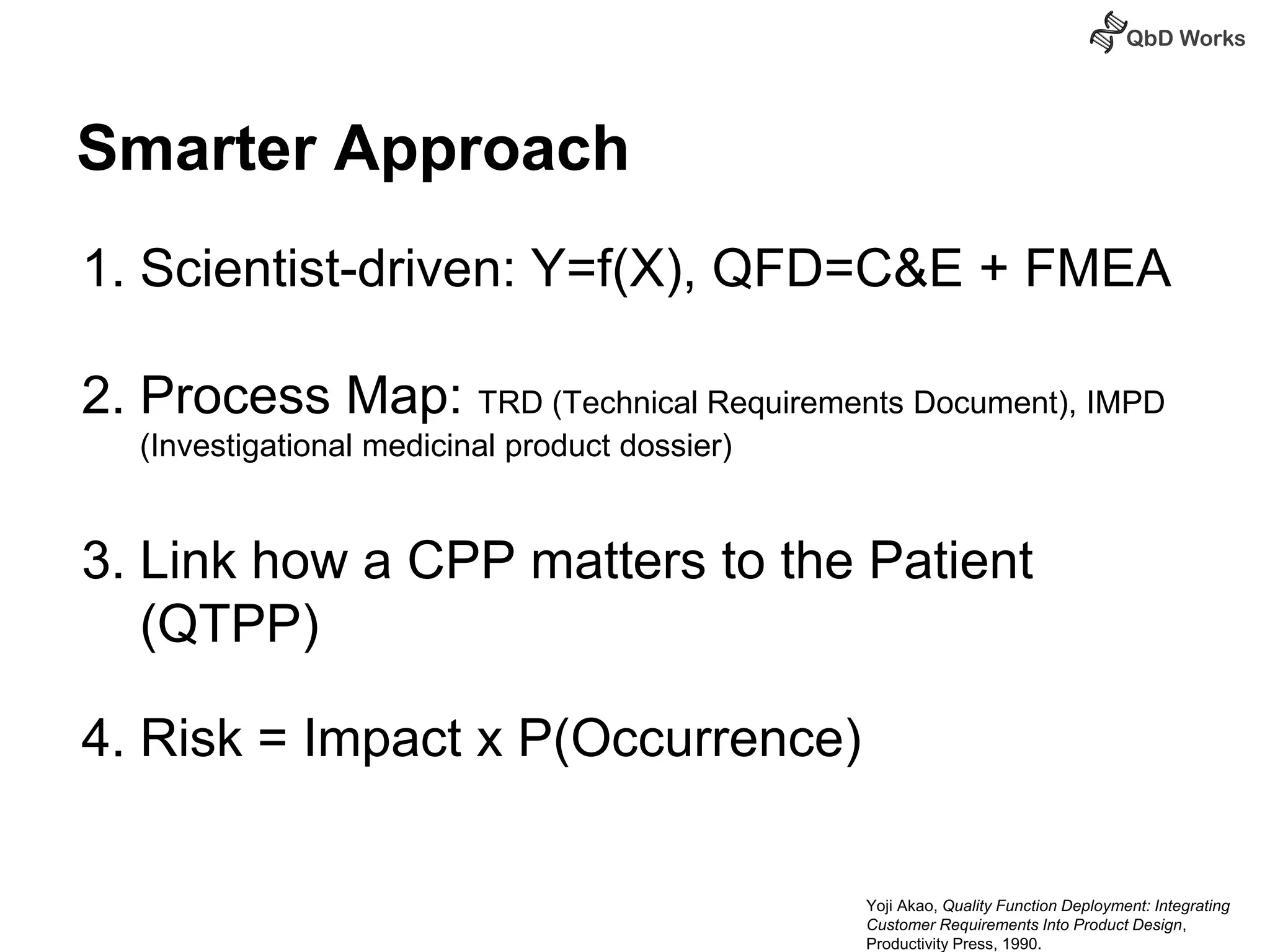
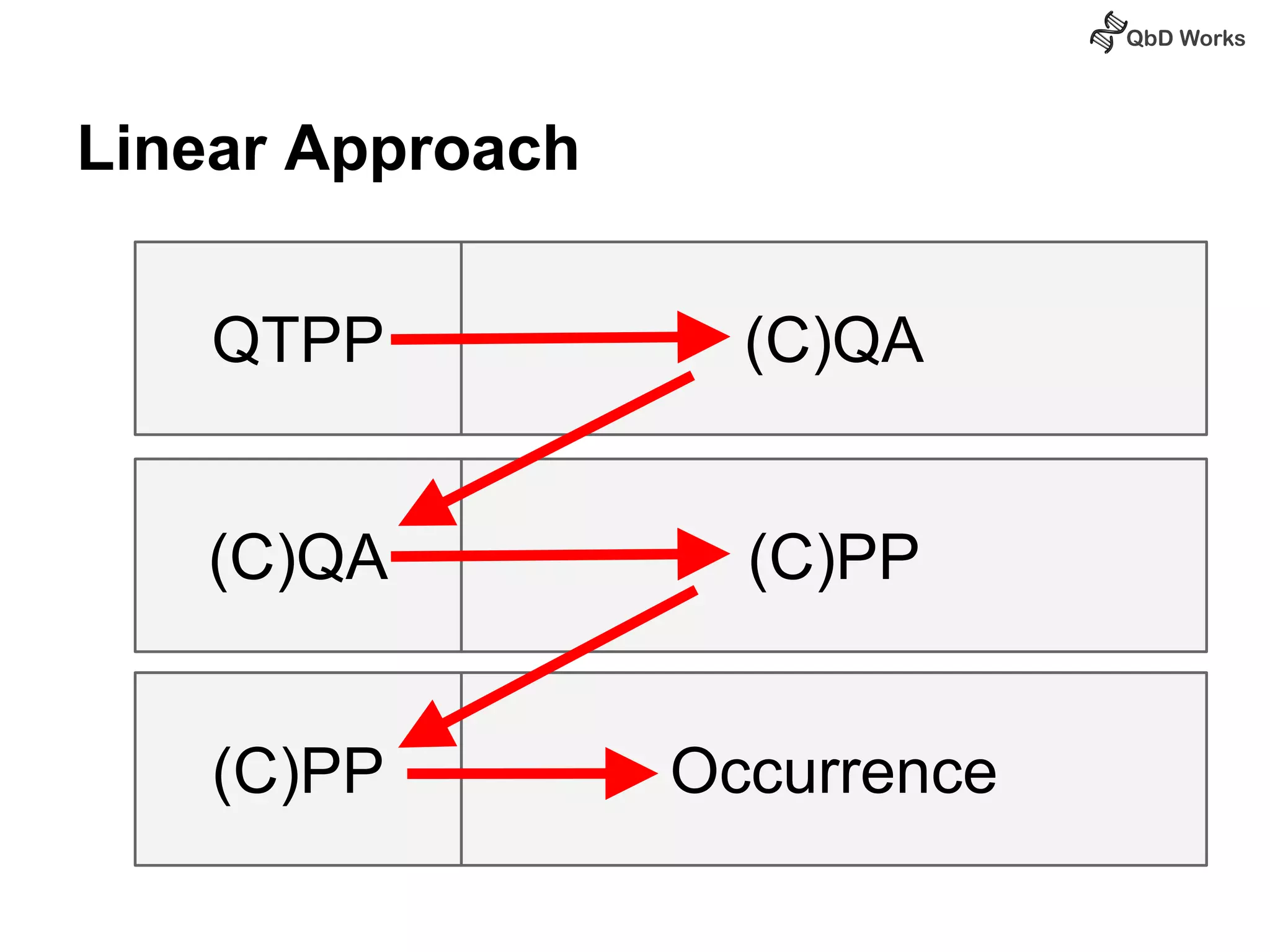
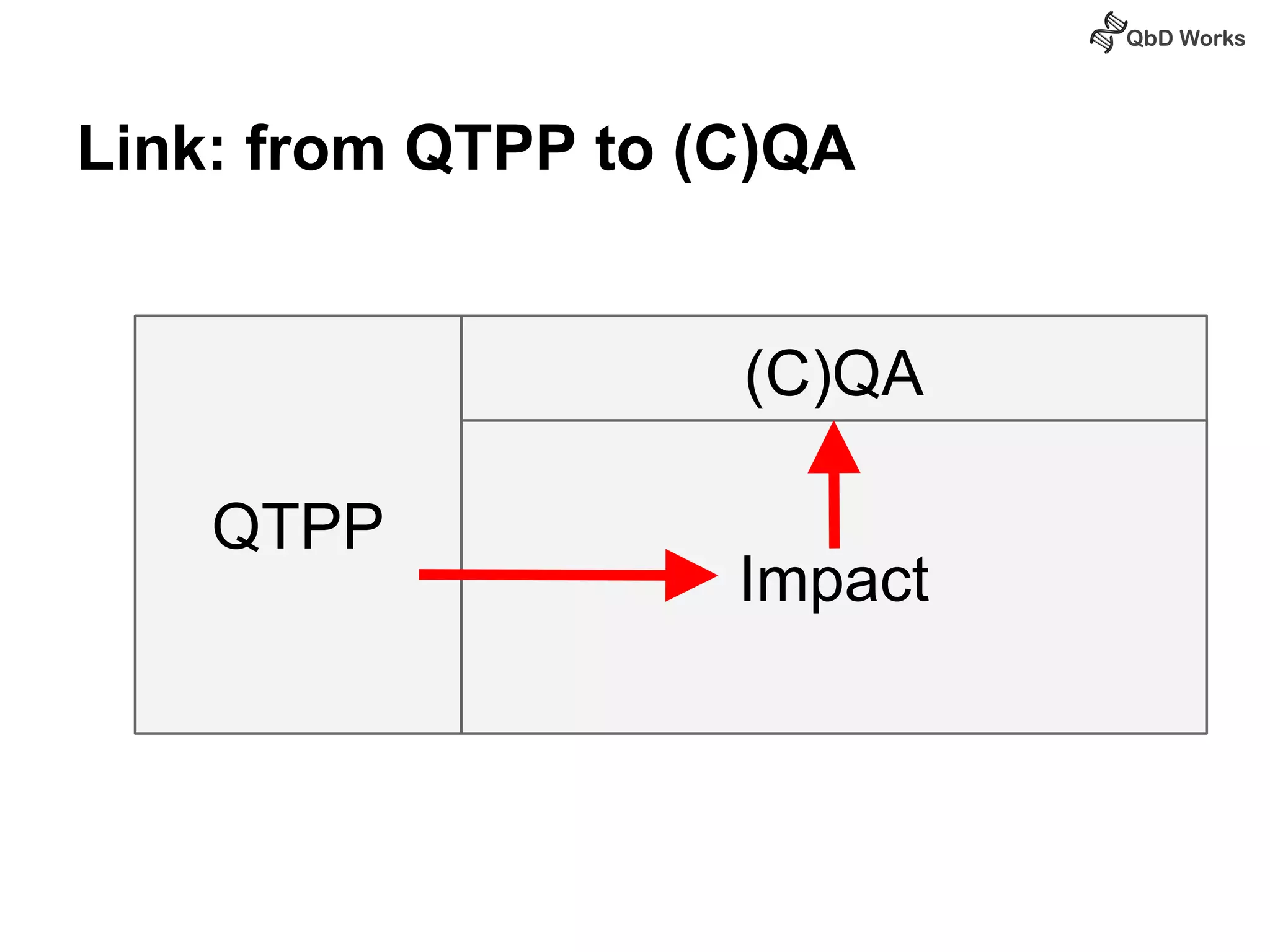
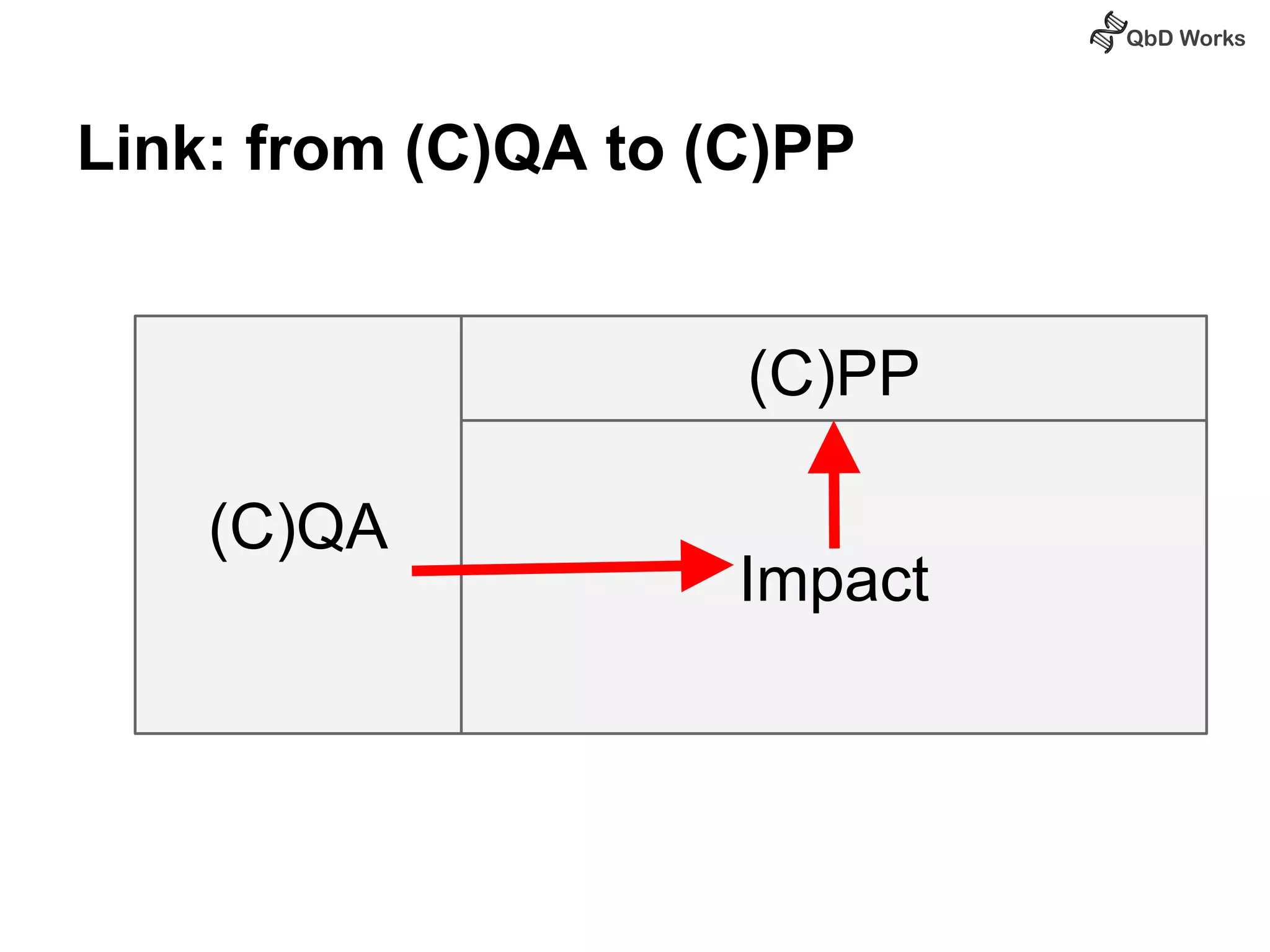
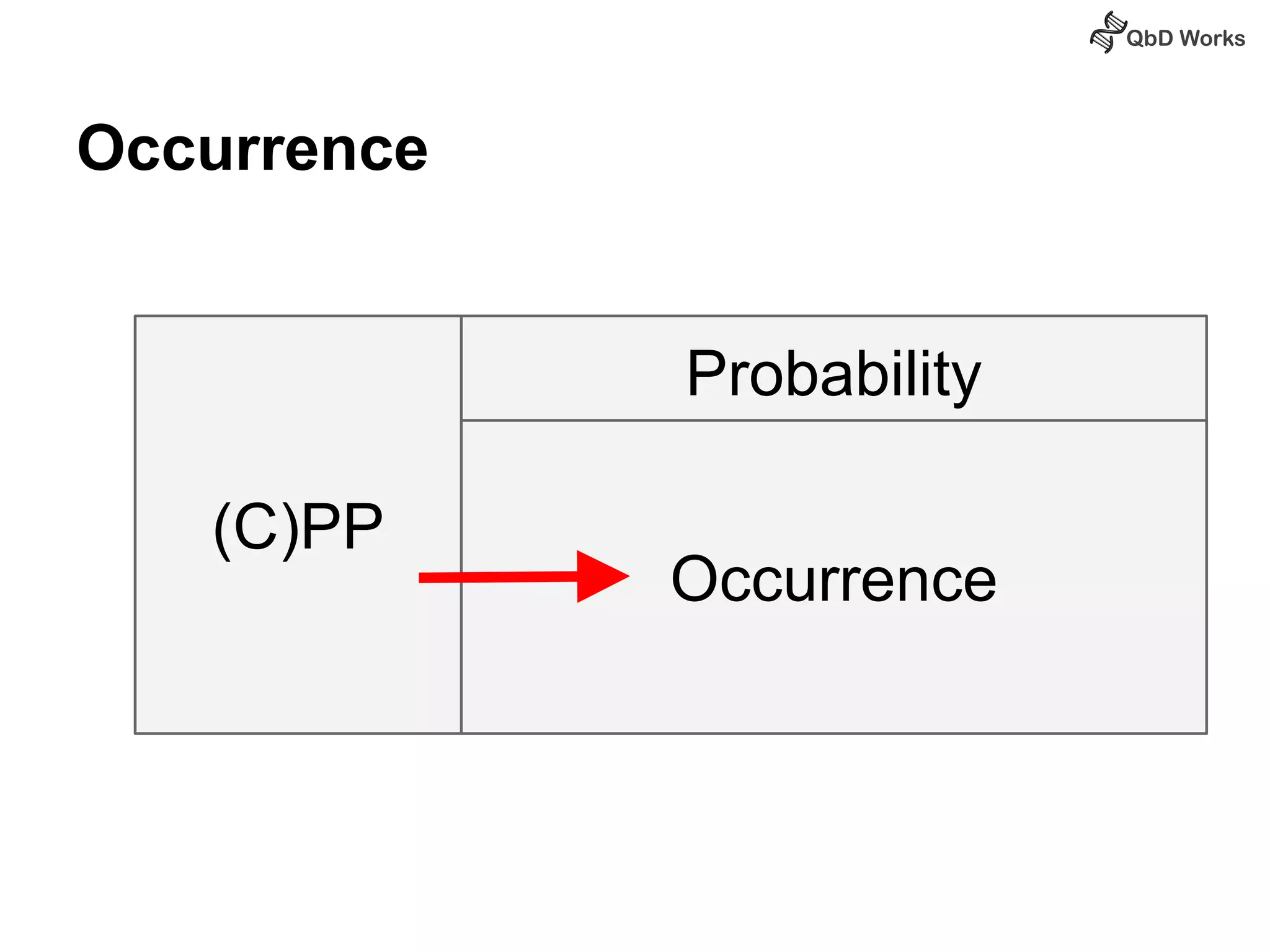
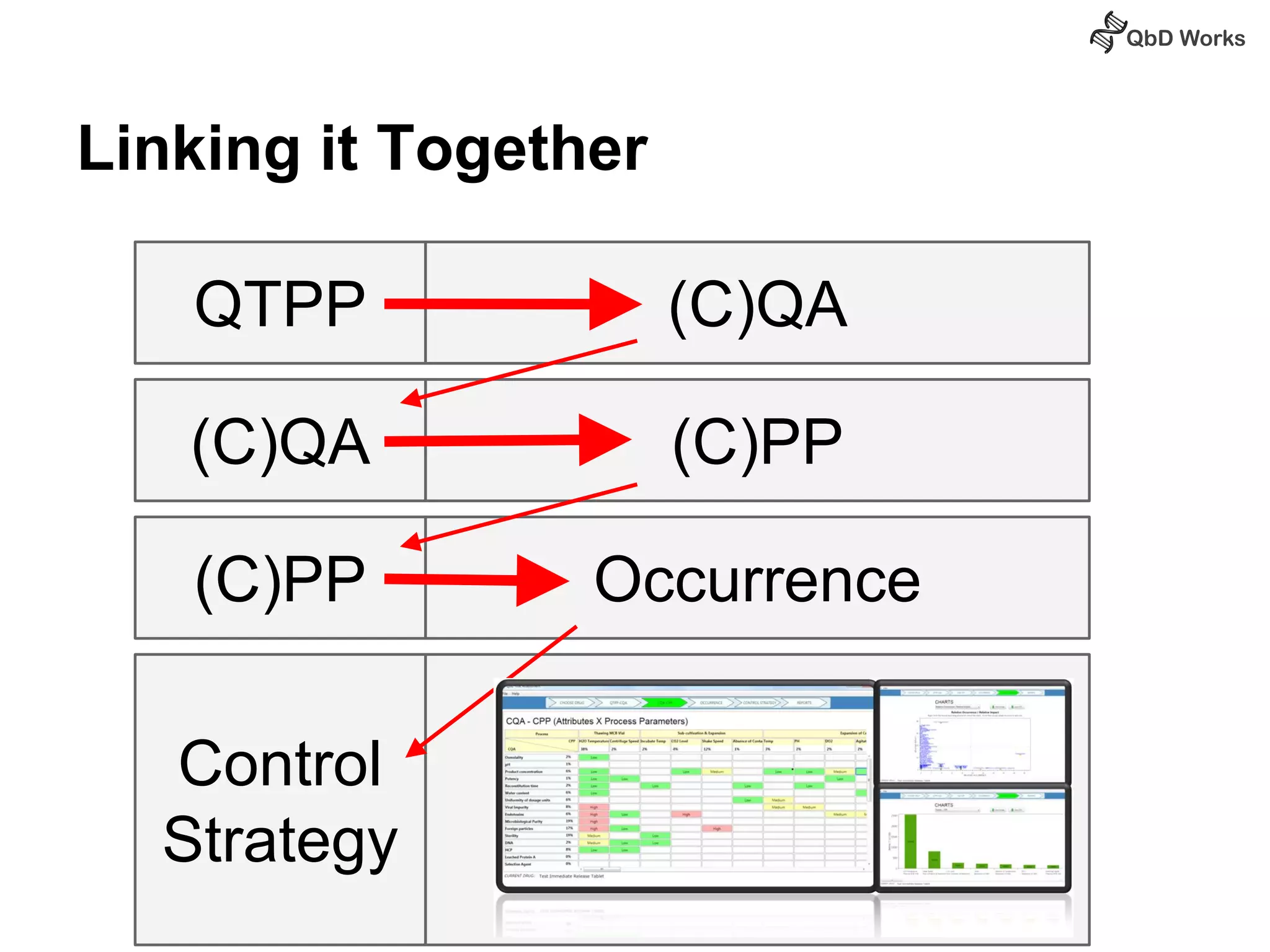
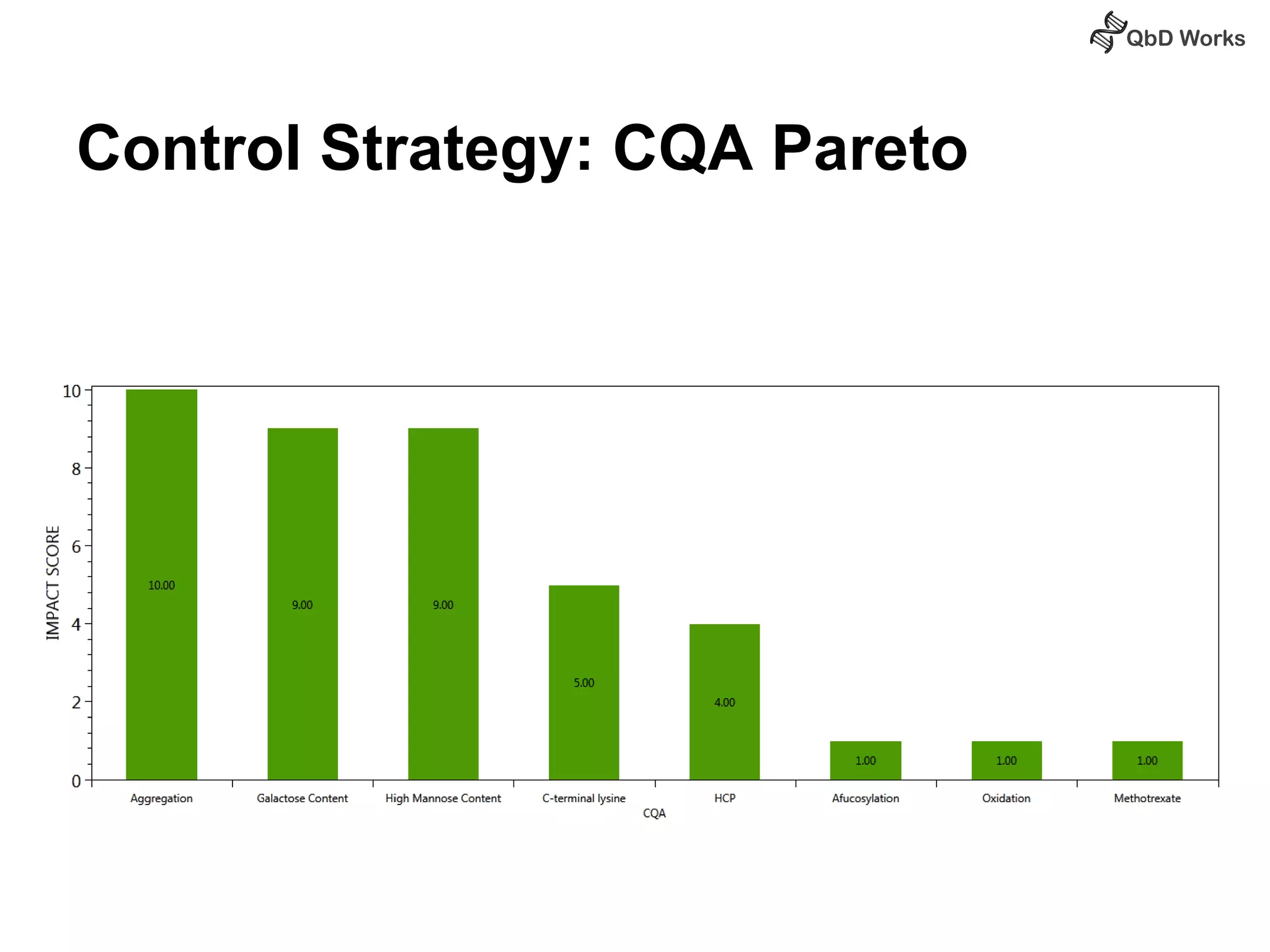
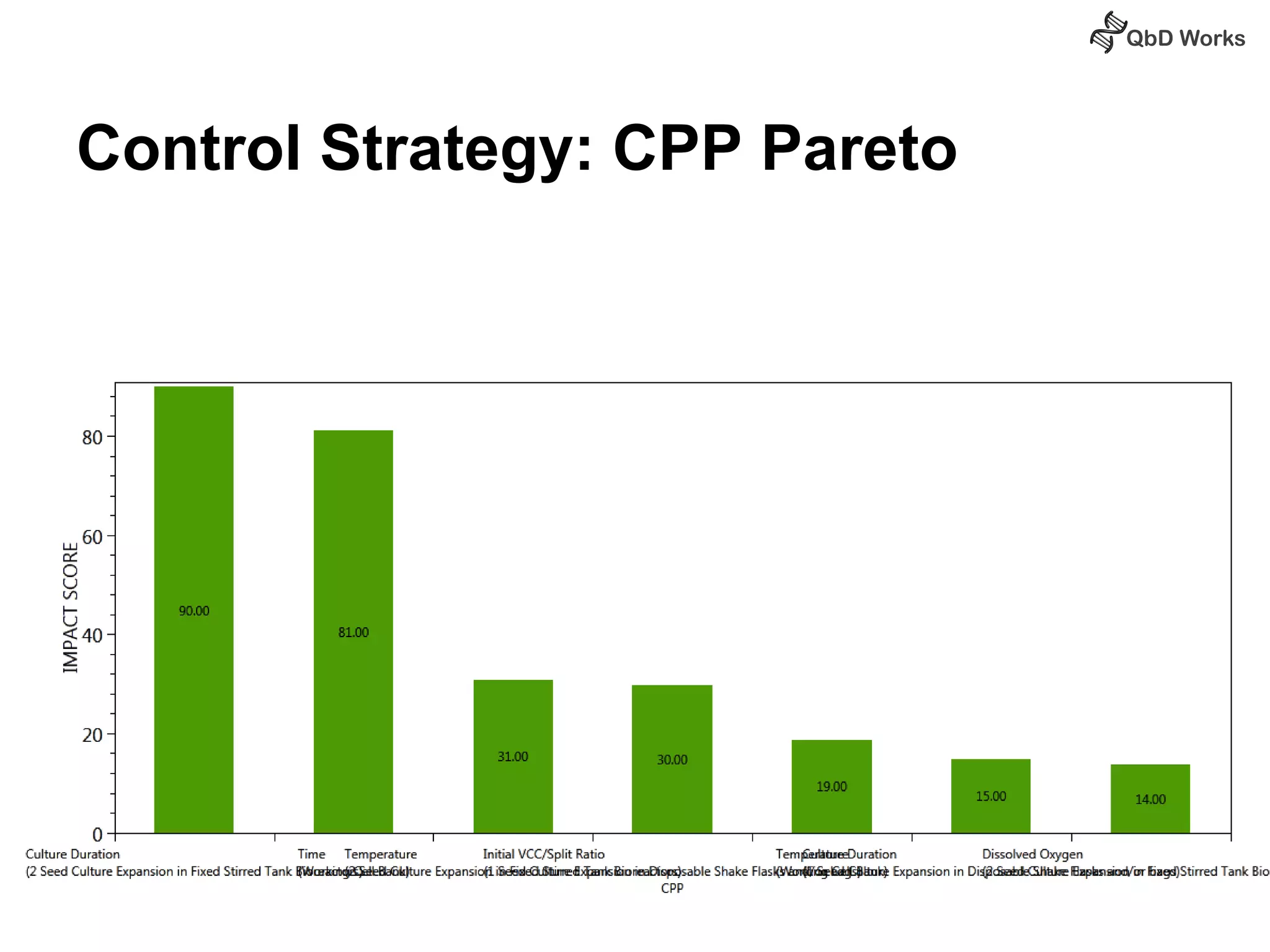
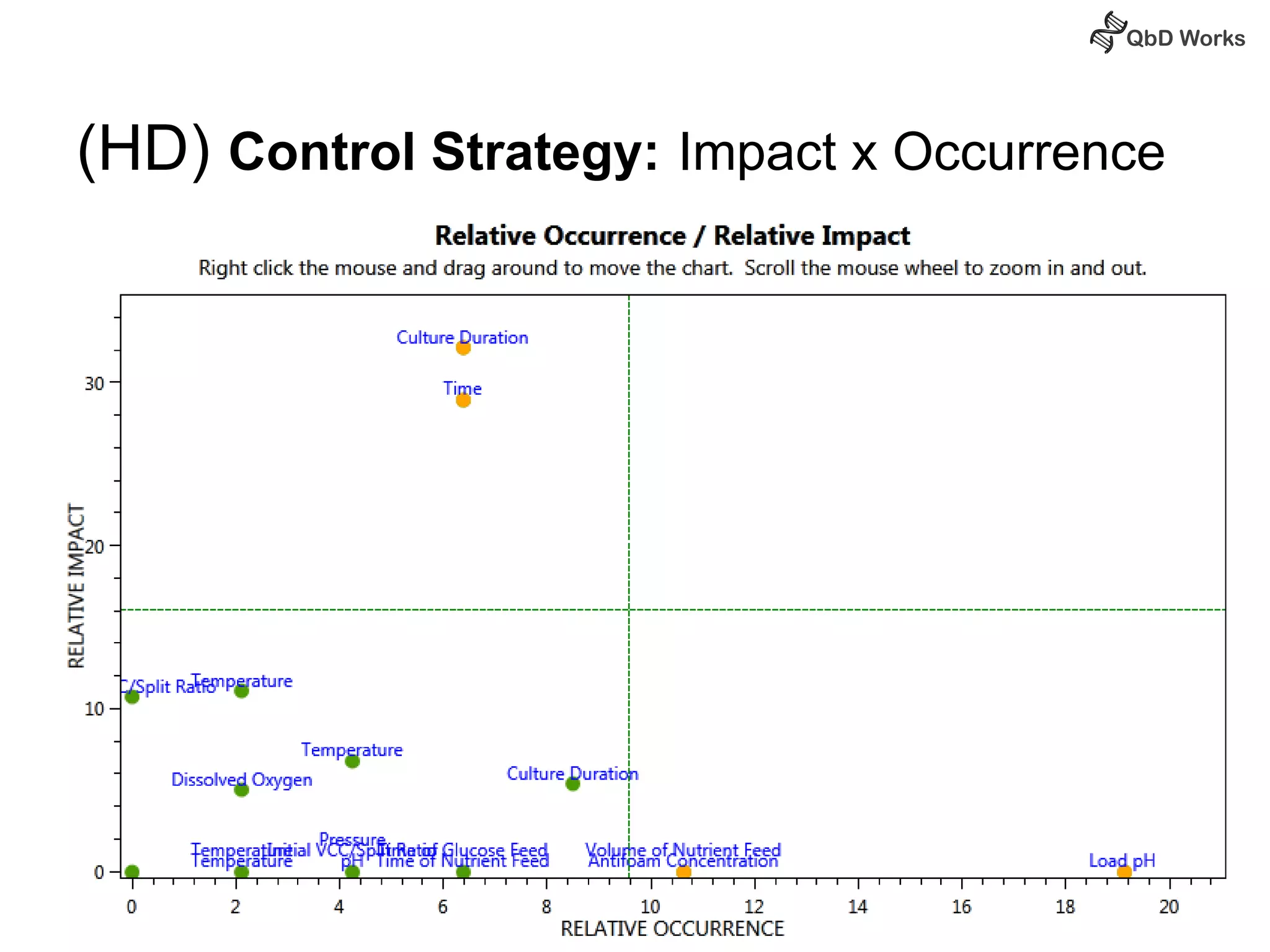
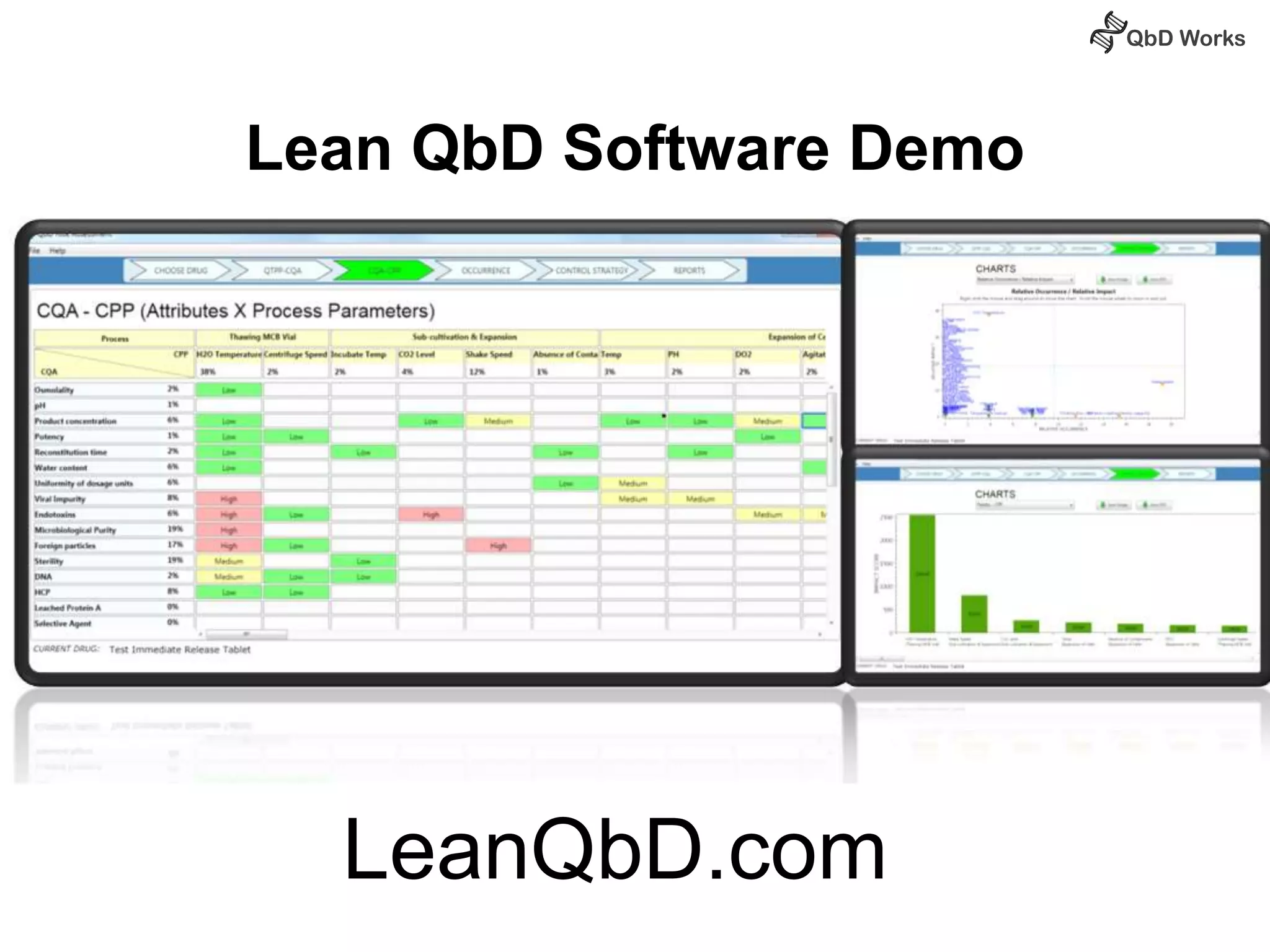
The document discusses best practices for risk assessment, which is the first step in Quality by Design (QbD). It recommends that risk assessment should focus on linking process parameters (CPP) to critical quality attributes (CQA) and how they ultimately impact the quality target product profile (QTPP). Failure Mode and Effects Analysis (FMEA) is not always the ideal tool for early development projects due to a lack of process understanding. Instead, it suggests using a simpler rating system of impact vs probability of occurrence to prioritize risks. Building these linkages from QTPP to CQA to CPP is key to developing a successful control strategy and executing QbD projects.