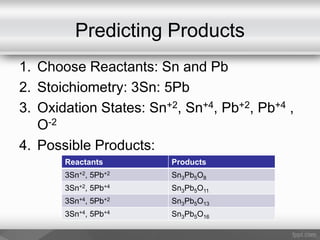
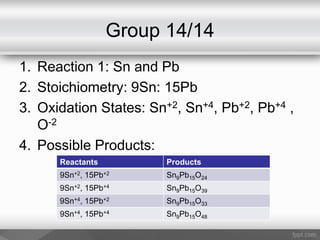
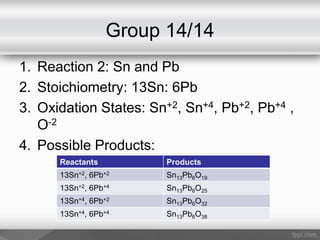
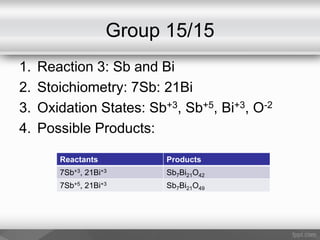
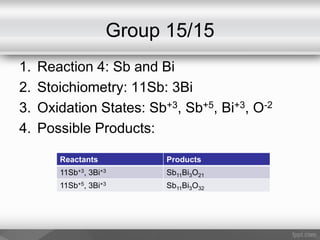
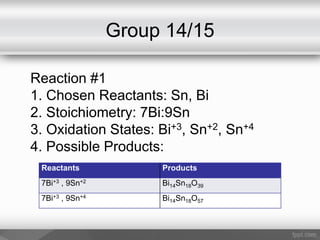
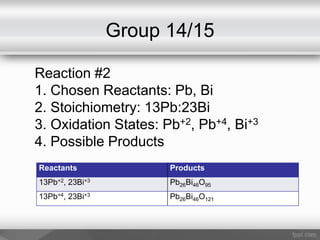
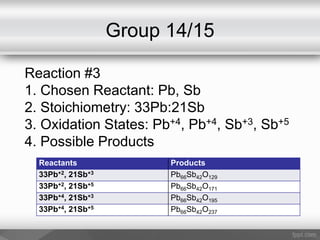
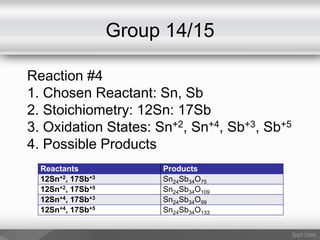
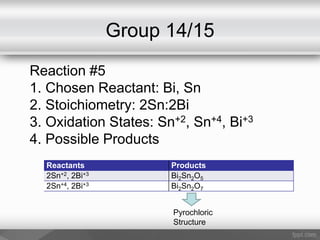
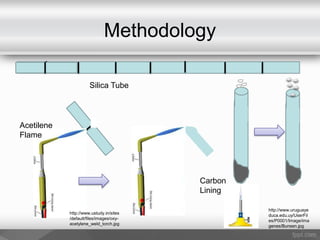
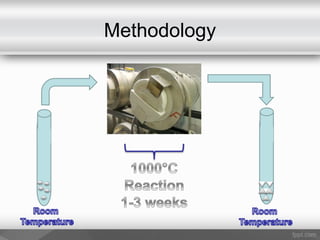
This document outlines a study on the solid-state synthesis of mixed-metal oxides. It discusses solid-state chemistry and synthesis, as well as technological applications of mixed-metal oxides. The authors propose synthesizing new mixed-metal oxide compounds from high-temperature reactions of solid powder reactants from groups 14/14, 14/15, and 15/15 on the periodic table. They predict possible products and outline a methodology using silica tubes, carbon lining, and high-temperature oven reactions. Limitations involving equipment and time are also noted, along with plans for future work synthesizing the proposed reactions.