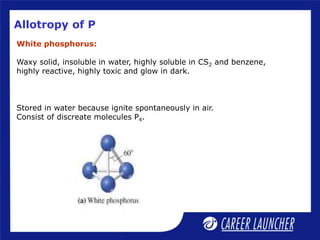
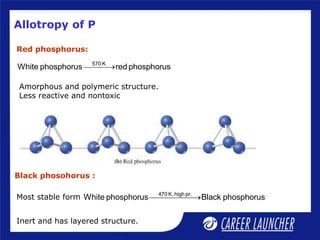
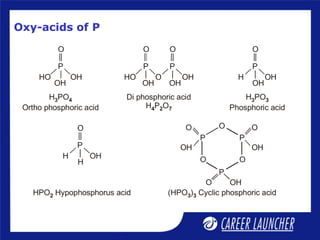
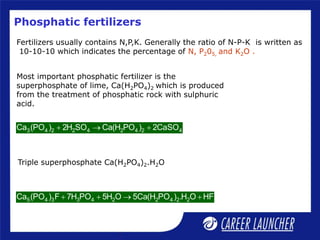
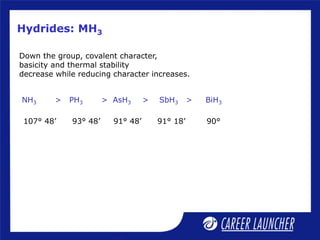
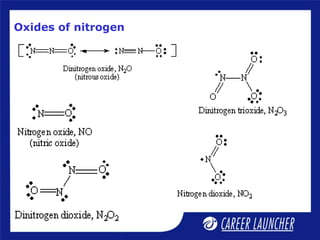
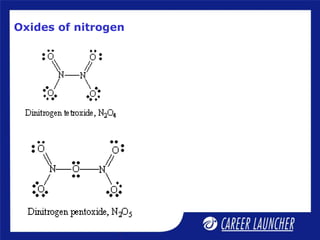
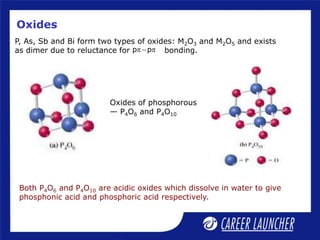
The document outlines the chemical properties and extraction methods for elements in Groups 14 and 15, including silicon, tin, and lead, as well as their reactions with acids and alkalies. It details the oxides, halides, and silicates of these elements, alongside their applications in various industries such as glassmaking and metallurgy. Additionally, it discusses the toxicity of lead and the significance of phosphatic fertilizers in agriculture.











![Amphiboles
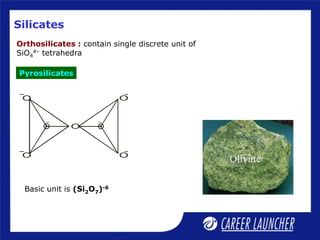
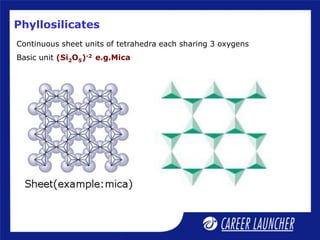
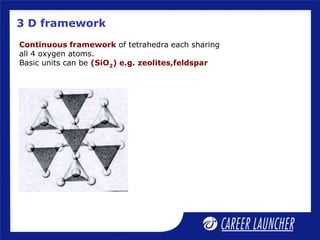
Continuous double chain units of tetrahedra
each sharing 2 and 3 oxygens alternately.
Basic unit is (Si4O11)-6 or (Si8O22)-12
e.g., asbestos; [Mg3(Si2O5)(OH)4]](https://image.slidesharecdn.com/dokumen-240625064655-e97cd2ec/85/dokumen-tips_51-p-block-elements-2-ppt-16-320.jpg)

![Group 15 elements
Nitrogen N [He] 2s2p3
Phosphorus P [Ne] 3s23p3
Arsenic As [Ar]3d104s24p3
Antimony Sb [Kr]4d105s25p3
Bismuth Bi [Xe]4f145d106s26p3](https://image.slidesharecdn.com/dokumen-240625064655-e97cd2ec/85/dokumen-tips_51-p-block-elements-2-ppt-21-320.jpg)






![Halides
Forms two series of halides;
MX3 (pyramidal)
MX5 (trigonal bipyramidal)
Trihalides readily hydrolyse with water.
3 2 4
NCl 4H O NH OH HOCl
3 2 3 3
PCl 3H O H PO 3HCl
3 2 3 3
AsCl 3H O H AsO 3HCl
3 2
SbCl H O SbO 3Cl 2H
3 2
BiCl H O BiO 3Cl 2H
PCl5 is molecular in gas and liquid phases but exists
as [PCl4]+[PCl6]- in the solid state .](https://image.slidesharecdn.com/dokumen-240625064655-e97cd2ec/85/dokumen-tips_51-p-block-elements-2-ppt-33-320.jpg)

![Illustrative Example
Solid phosphorous-pentachloride exhibits some
ionic character, why?
Solution
This is because PCl5 exists as [PCl4]+ [PCl6]- in solid
phase and hence exhibits ionic character.](https://image.slidesharecdn.com/dokumen-240625064655-e97cd2ec/85/dokumen-tips_51-p-block-elements-2-ppt-35-320.jpg)