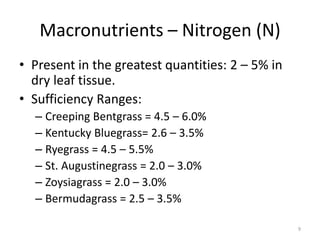
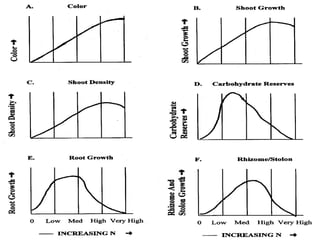
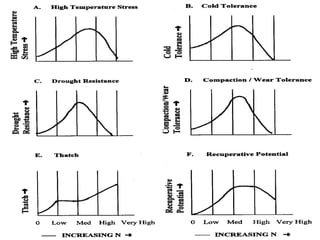
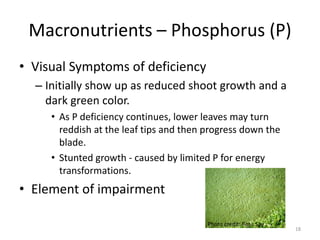
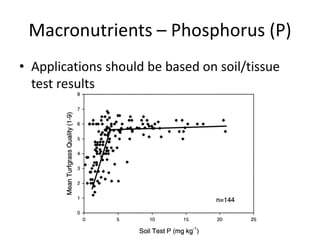
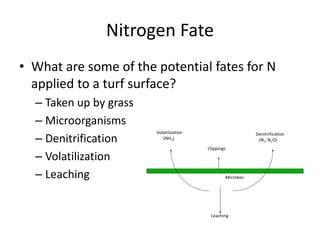
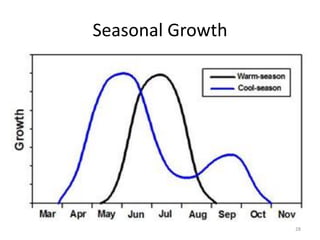
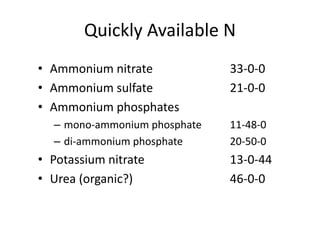
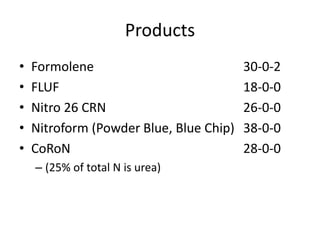
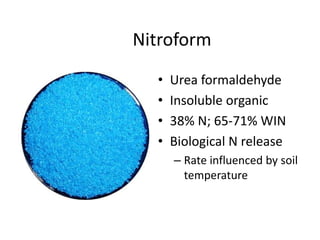
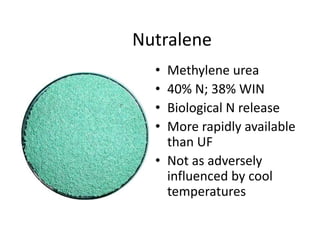
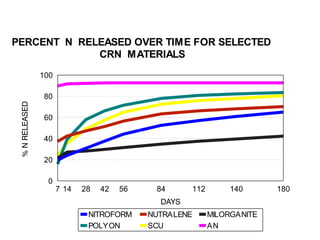
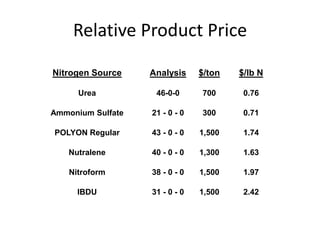
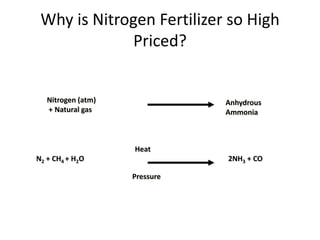
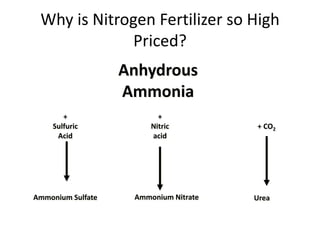
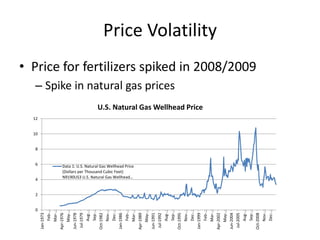
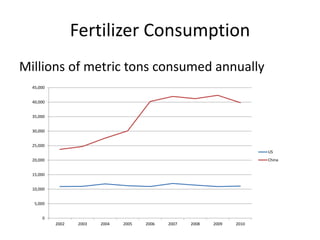
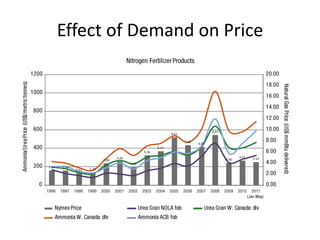
The document discusses the challenges facing turf management, particularly rising fertilizer prices and the need for effective nutrient management amidst reduced budgets and increased demands. It emphasizes the importance of understanding plant nutrition, specifically macronutrients like nitrogen, phosphorus, and potassium, along with their deficiencies and impact on turf health. Additionally, it presents various fertilizer options and management strategies to optimize performance and costs in turfgrass maintenance.