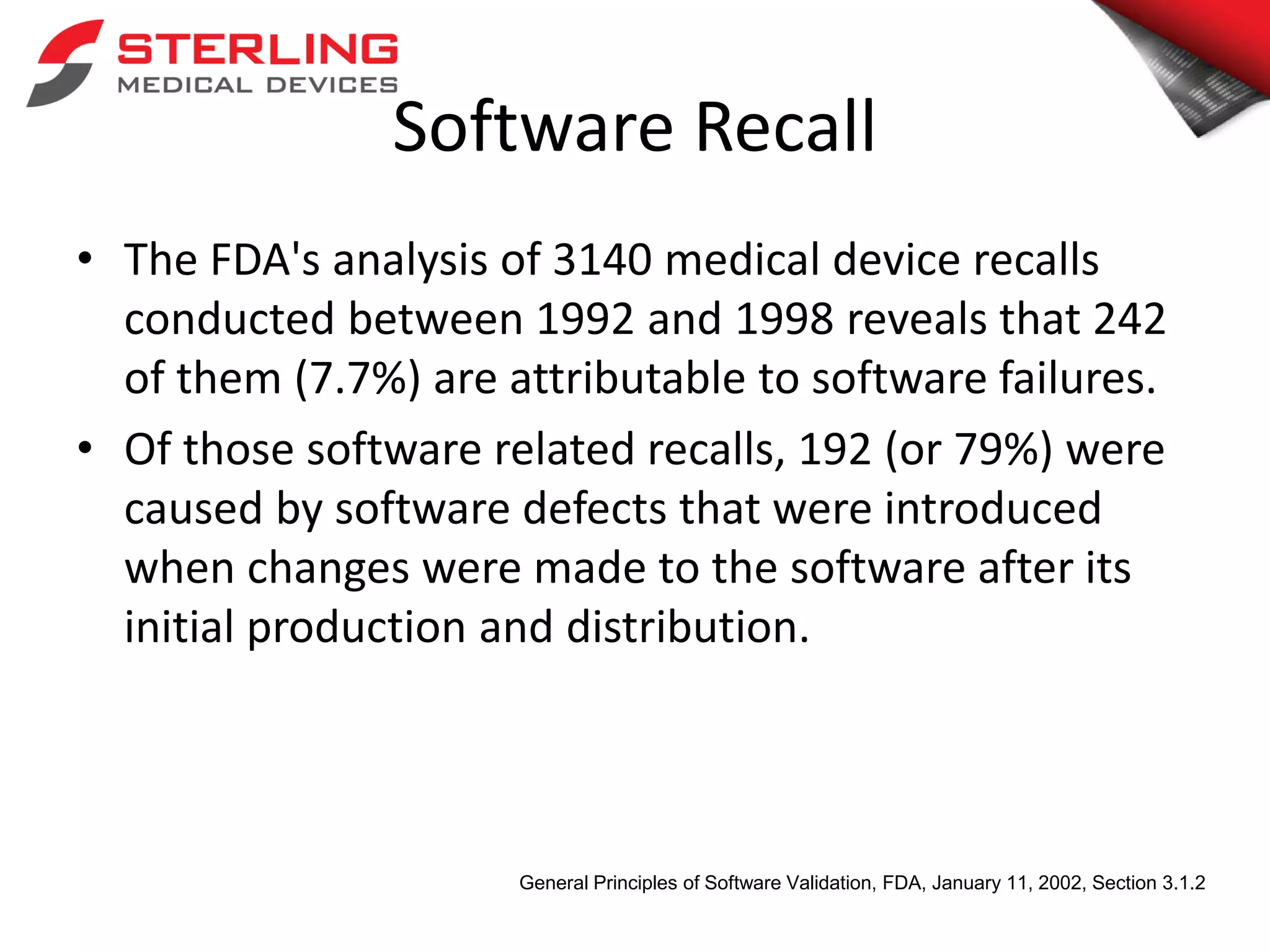
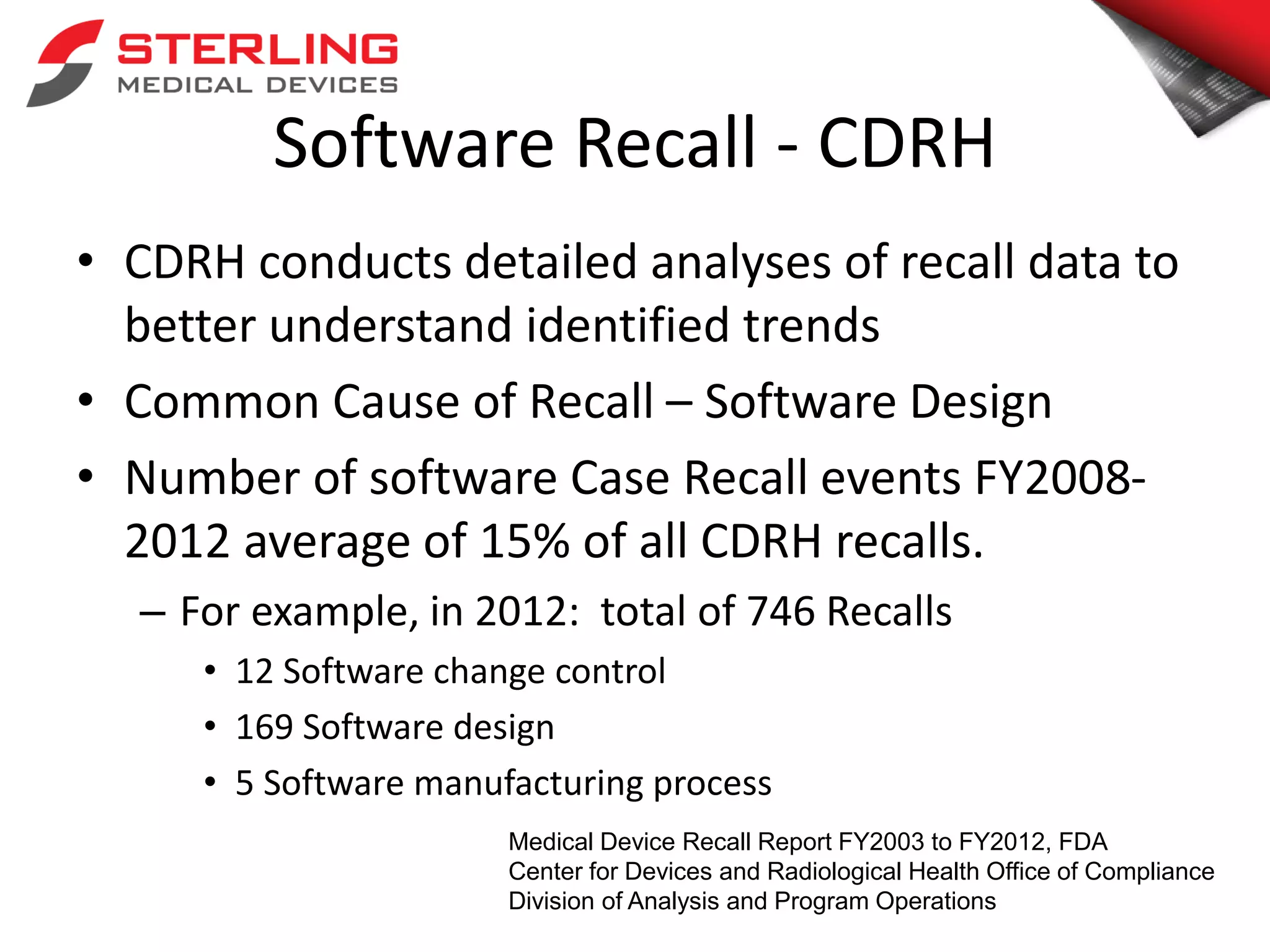
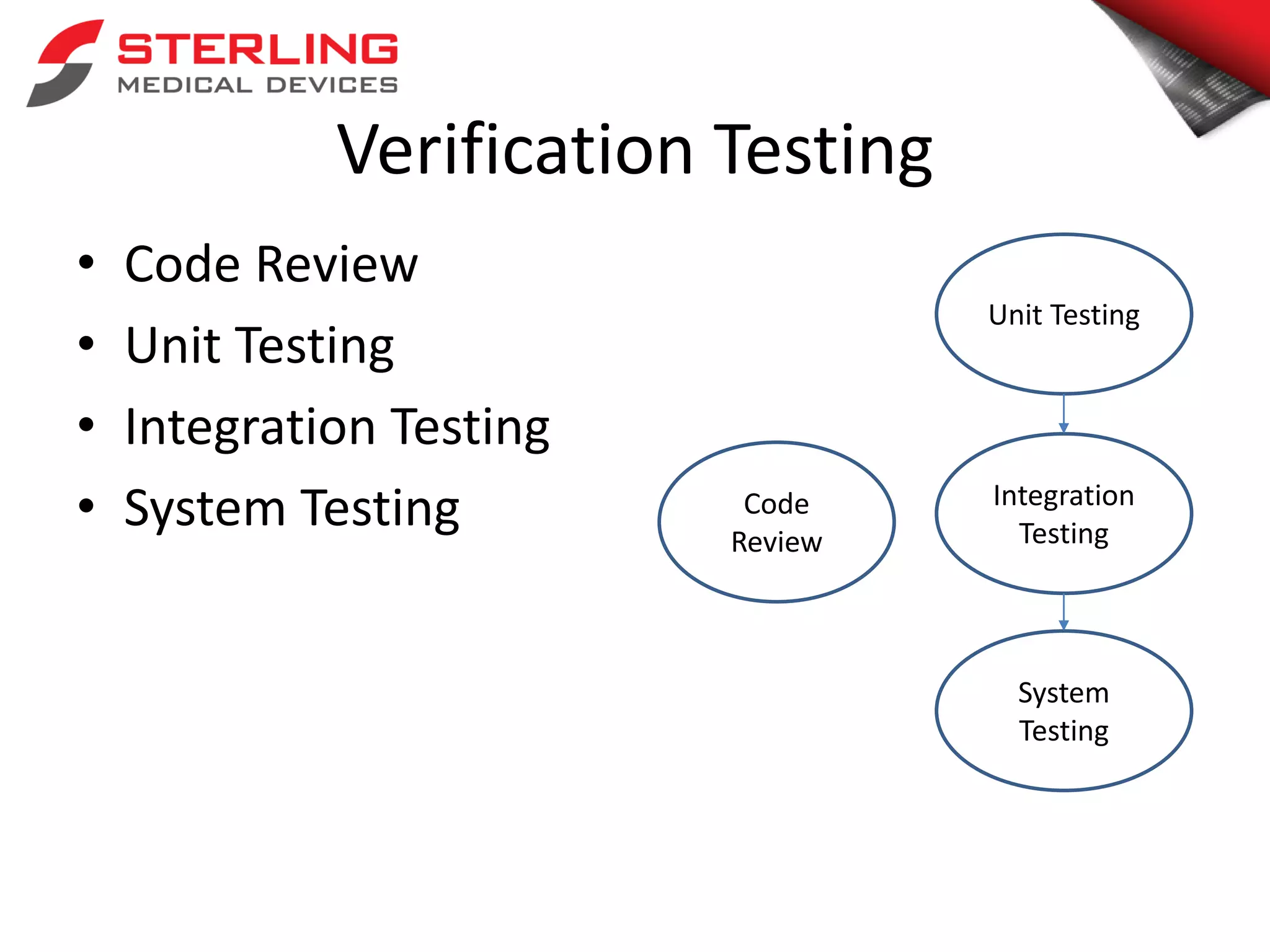
The document discusses the critical role of verification and validation (V&V) in medical device software development, highlighting that improper V&V can lead to software failures, recalls, and implications for device functionality. It outlines the definition of verification and validation, their differences, and types of testing, while citing FDA statistics on software-related recalls. The conclusion emphasizes that effective V&V processes are essential for ensuring user needs are met and for maintaining the safety of medical devices.