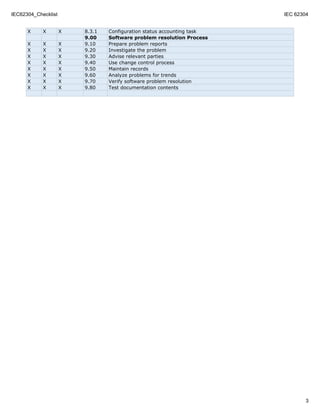
This document outlines the requirements for software life cycle processes according to IEC 62304. It lists the requirements for each class of medical device (A, B, C) for various chapters including software development processes, risk management, configuration management, and problem resolution. Requirements range from planning and documentation to implementation, integration testing, verification, and maintenance for the different classes.