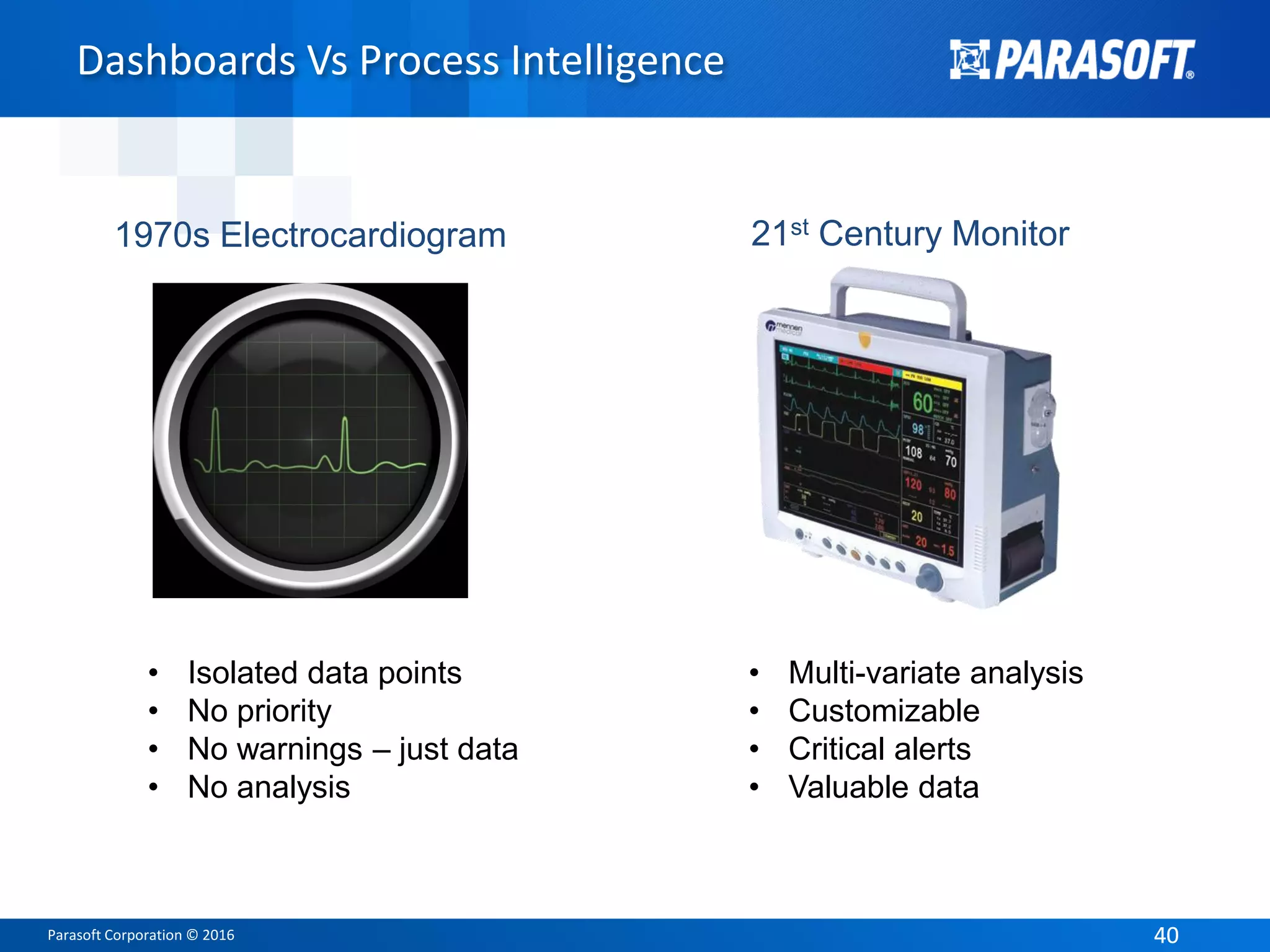
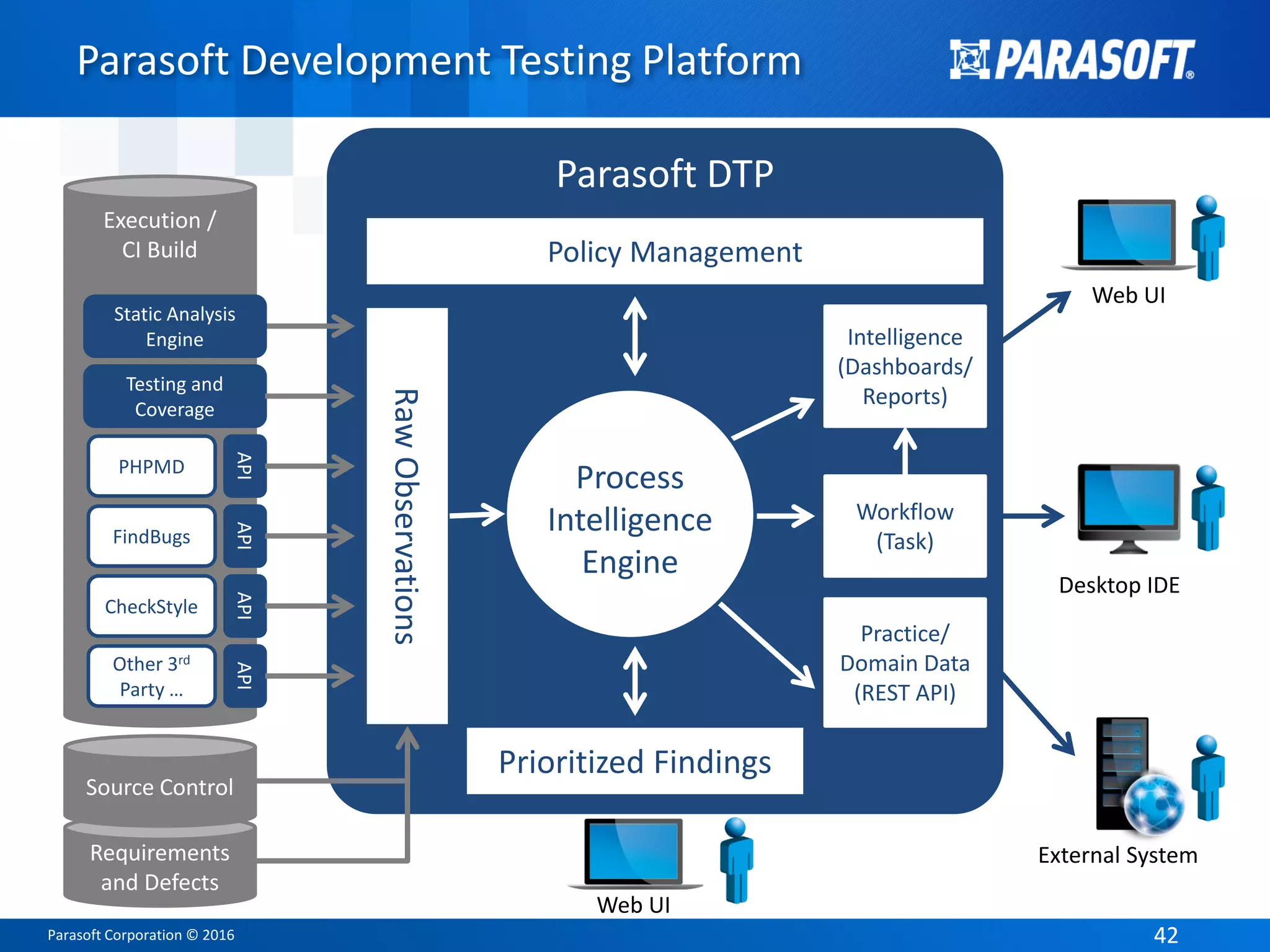
The document presents an overview of FDA software compliance for medical devices and emphasizes the importance of static analysis in adhering to regulatory standards such as IEC 62304. It highlights the roles of presenters Arthur Hicken and Kelly Weyrauch, who share their expertise in software development, quality management, and regulatory processes. The document outlines key FDA regulations and guidance documents while advocating for best practices in software development to ensure safety and efficacy in medical devices.



























![Parasoft Corporation © 2016 3939
Sample Rules for FDA Static Analysis
Avoid accessing arrays out of bounds
Avoid use before initialization
Avoid null pointer dereferencing
Avoid overflows due to [various causes]
Avoid division by zero
Ensure deallocation functions guarantee resource
freeing
Do not use resources that have been freed
Do not free resources using invalid pointers
Do not abandon unreleased locks
Do not use blocking functions while holding a lock
Ensure resources are freed
Do not abandon unreleased locks
Properly terminate character strings
Never return a reference to a local object](https://image.slidesharecdn.com/rxforfdasoftwarecompliance-160923234553/75/Rx-for-FDA-Software-Compliance-37-2048.jpg)