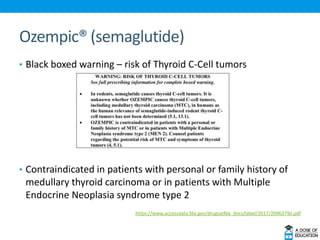
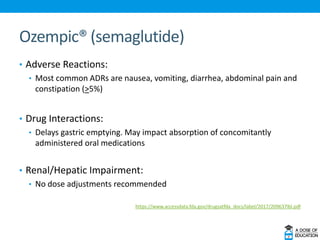
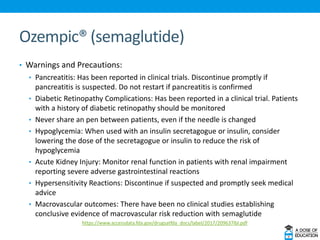
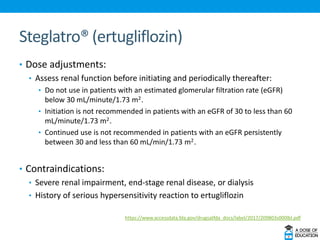
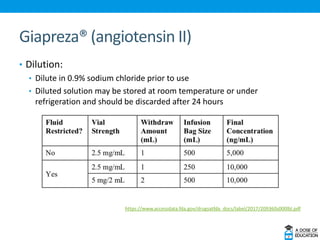
Ozempic (semaglutide) was approved for type 2 diabetes. It is administered once weekly and starts at 0.25 mg, increasing to 0.5 mg after 4 weeks and 1 mg if needed. Common side effects include nausea and vomiting. Ertugliflozin (Steglatro) was approved for type 2 diabetes to improve glycemic control. It is dosed once daily at 5 or 15 mg. Angiotensin II (Giapreza) was approved to increase blood pressure in adults with septic or distributive shock. It is administered as an IV infusion. Netarsudil (Rhopressa) was approved for glaucoma or ocular hypertension