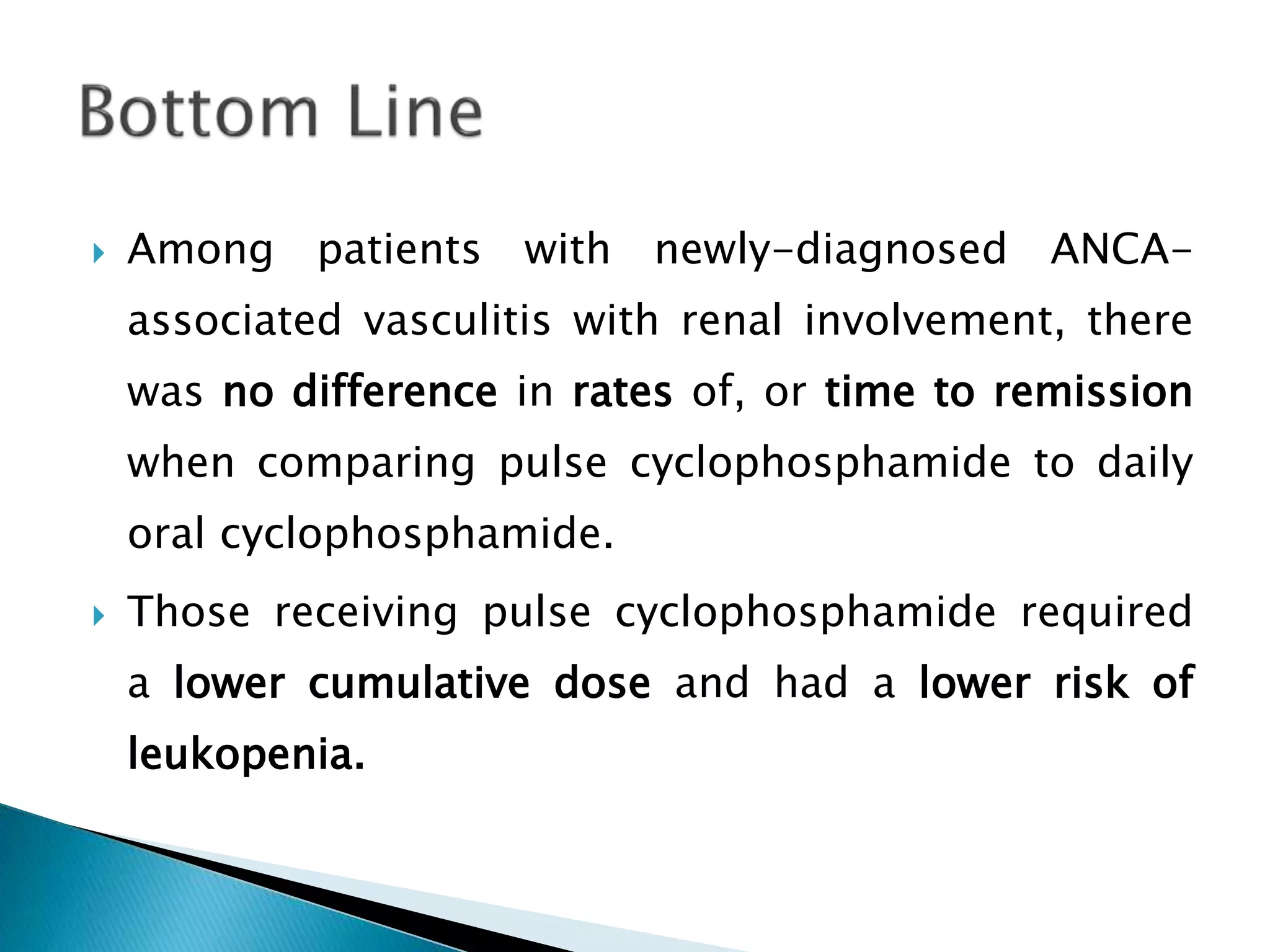
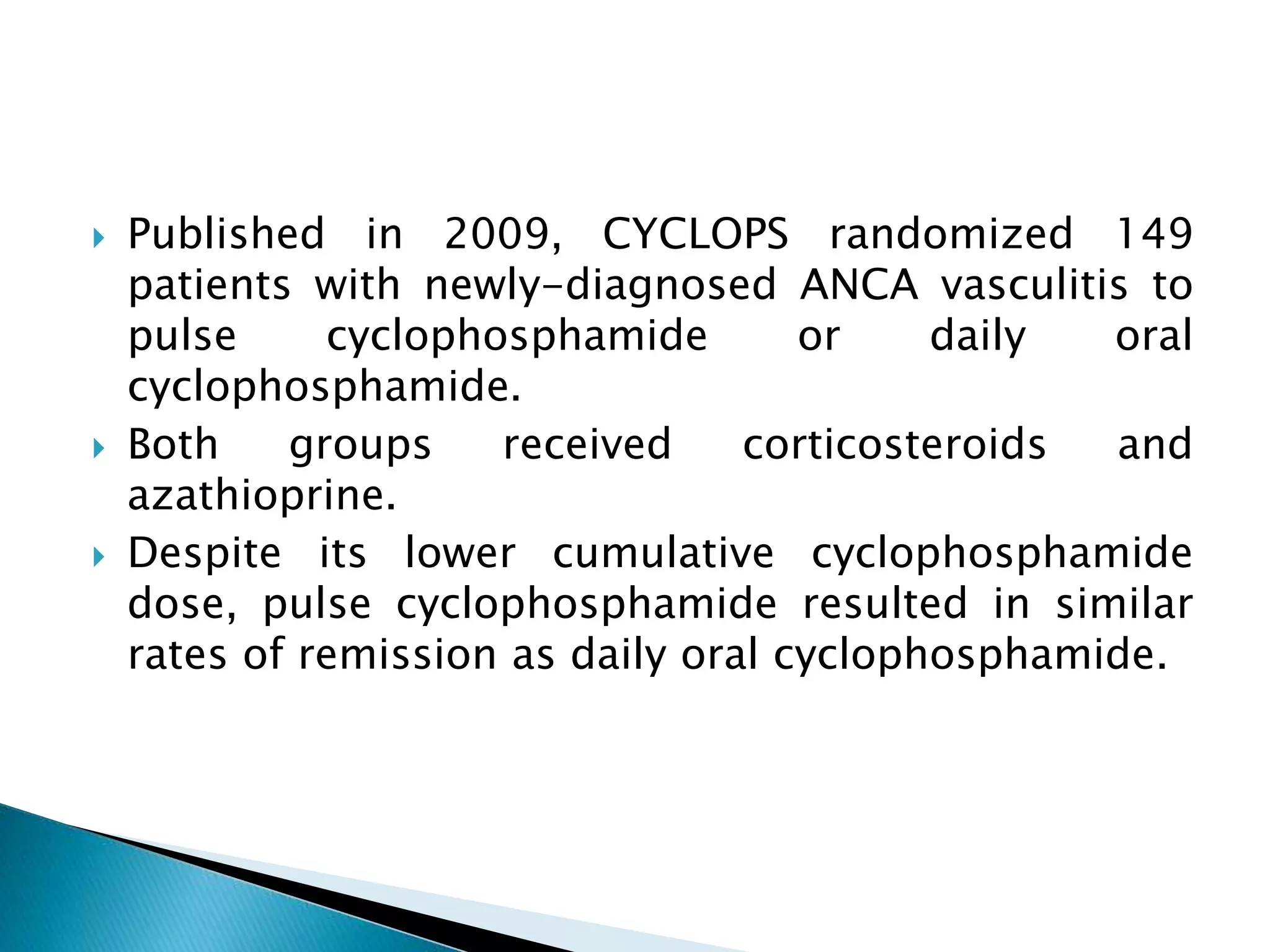
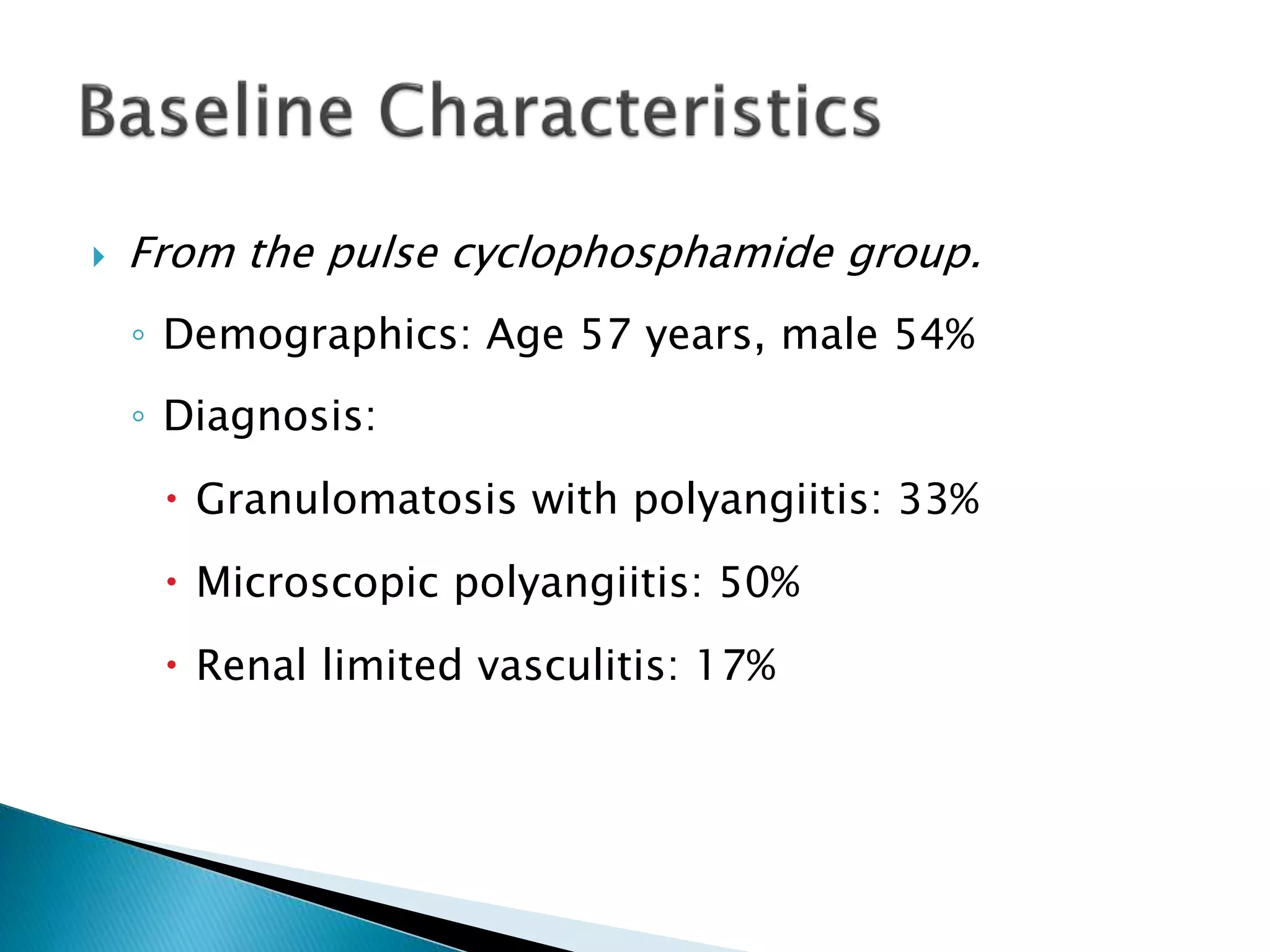
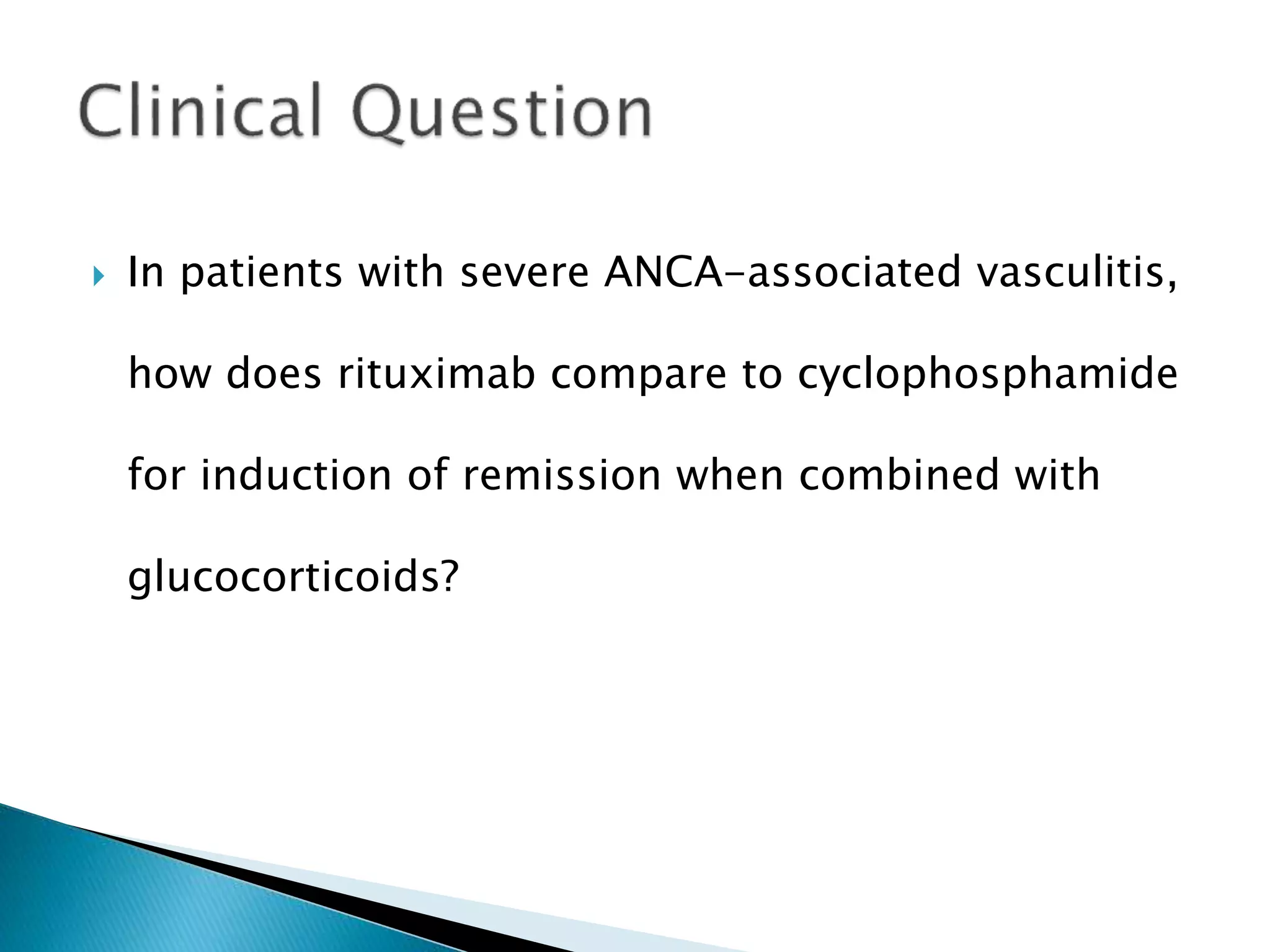
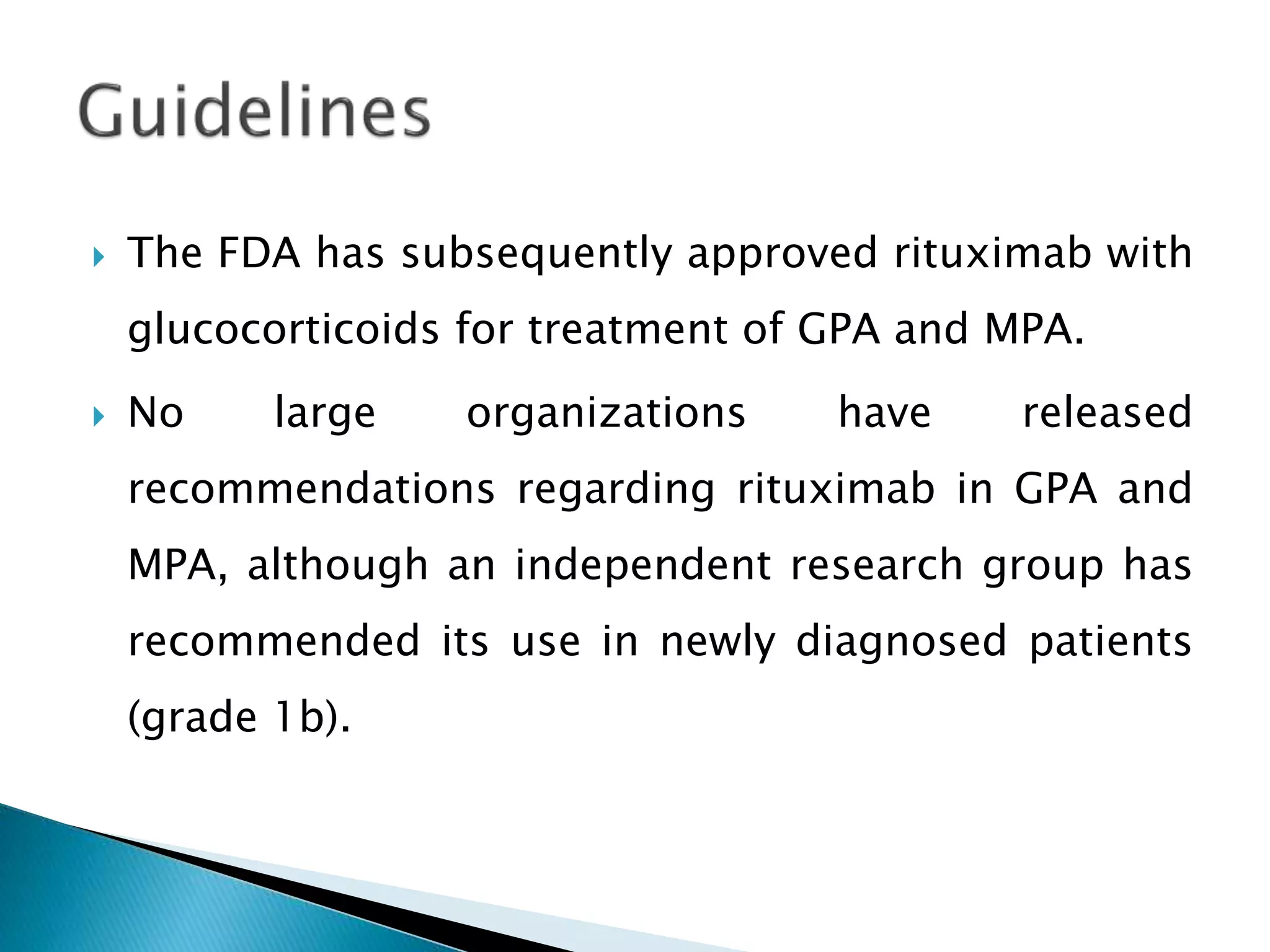
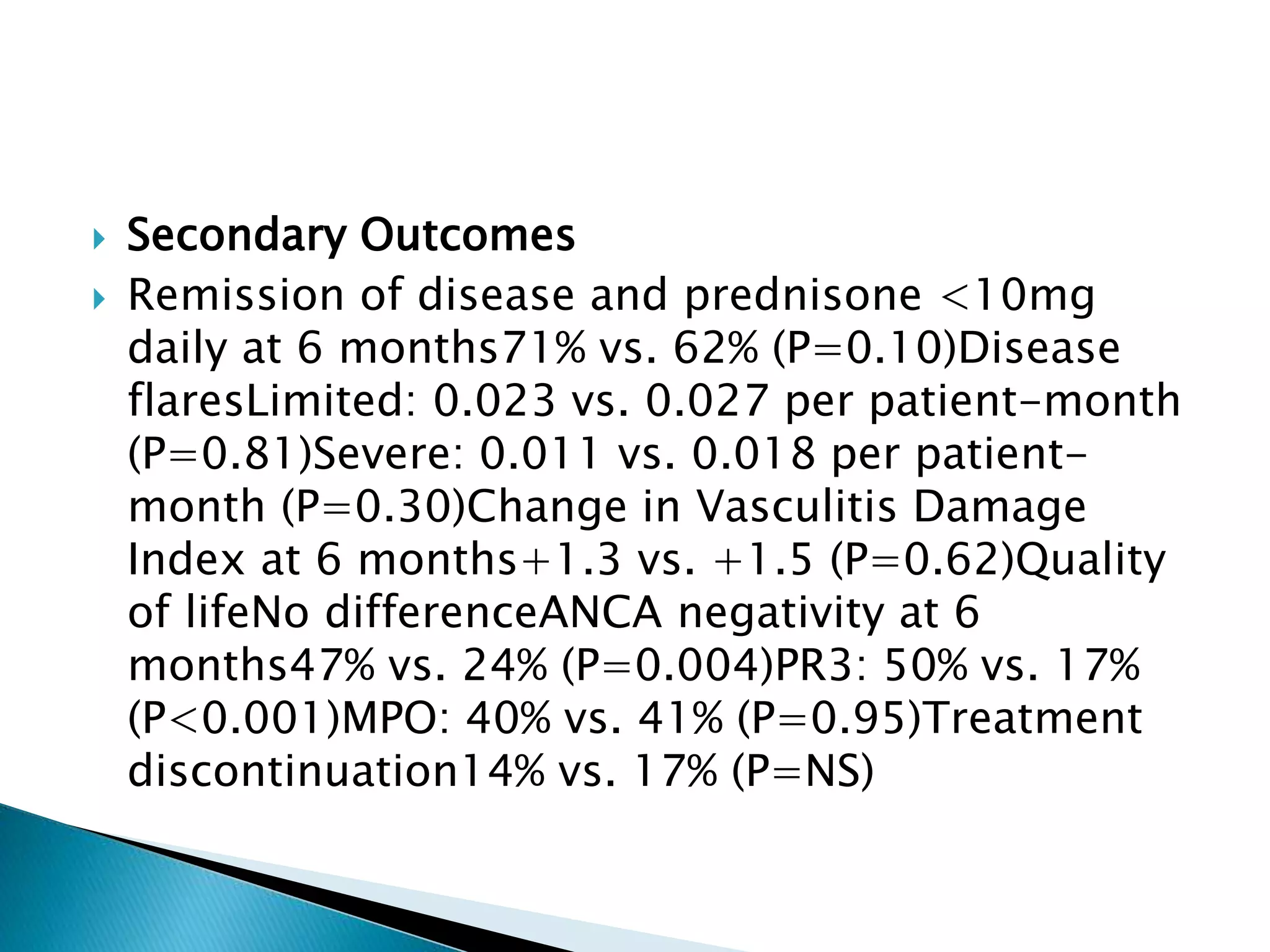
This document summarizes a randomized controlled trial comparing rituximab to cyclophosphamide for inducing remission in patients with severe ANCA-associated vasculitis. The trial found rituximab to be non-inferior to cyclophosphamide at inducing remission at 6 months when both were combined with glucocorticoids. Rituximab achieved similar rates of remission and disease control as cyclophosphamide with comparable safety profiles. Based on these results, rituximab was subsequently approved by the FDA for treatment of granulomatosis with polyangiitis and microscopic polyangiitis.