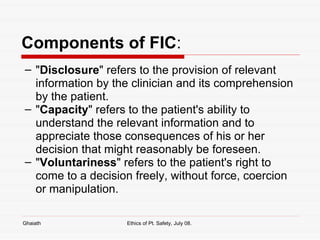
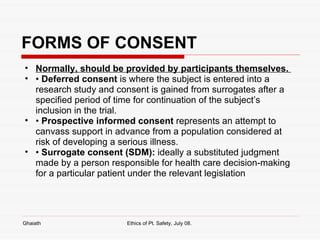
This document discusses various bioethical issues related to patient safety in clinical practice and research. It begins by defining bioethics and describing when an issue becomes an ethical one. It then discusses key principles like autonomy, beneficence and justice. Specific issues covered include informed consent and its components, disclosure, capacity, voluntariness and exceptions. Research ethics guidelines and principles are also summarized, including issues like clinical equipoise, dual role of doctors, informed consent in research, and managing conflicts of interest.