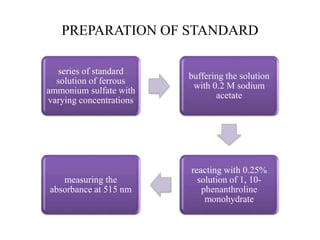
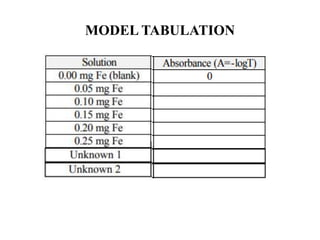
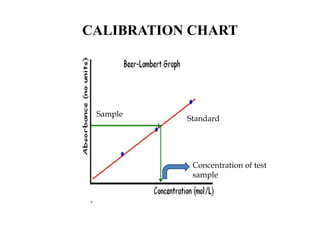
This document describes a method to estimate the iron content in a sample using 1,10-phenanthroline. Iron (II) forms an orange-red complex and iron (III) forms a yellow complex with the reagent. Both complexes can be determined by measuring absorbance. A test sample is made acidic and reacted with the reagent. Absorbance at 396nm gives total iron while 515nm gives iron(II). Standards of known iron concentration are prepared and a calibration curve of absorbance vs. concentration is generated. The curve is used to determine the iron concentration in unknown samples from their absorbance.

![• Iron content in the sample can be determined by
complexing with 1, 10-phenanthroline.
• Iron (II) forms an orange red color complex [Fe
(C12H8N2)3]2+
• iron (III) forms yellow color complex with the same
reagent [Fe (C12H8N2)3]3+.
• Both iron (II) and (III) can be determined from the
absorbance of the complexes.](https://image.slidesharecdn.com/estimationofiron-230109093005-e24c9589/85/ESTIMATION-OF-IRON-pptx-2-320.jpg)