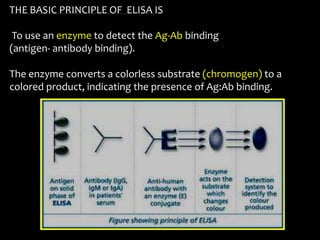
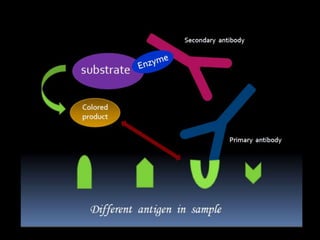
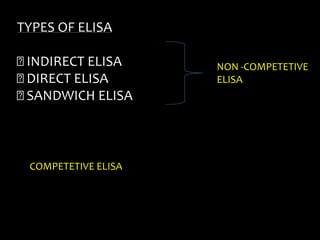
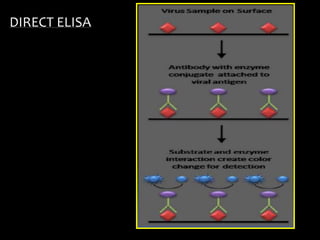
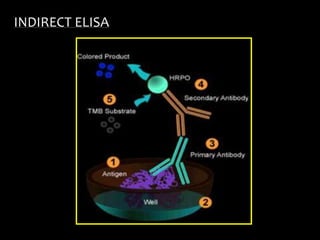
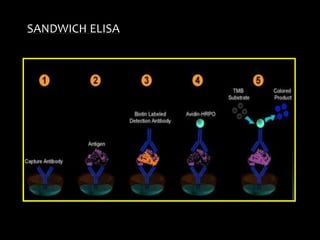
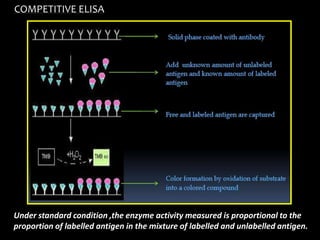
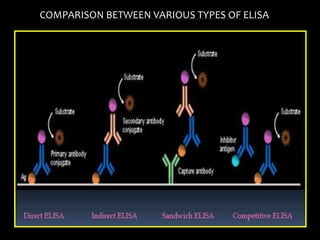
The document summarizes ELISA (enzyme-linked immunosorbent assay), a common analytical biochemistry technique. It describes the history, basic principles, components and types of ELISA including indirect, direct, sandwich and competitive ELISA. The basic principle is to use an enzyme to detect antigen-antibody binding by having the enzyme convert a colorless substrate to a colored product, indicating the presence of binding. Common applications are screening blood donations for viruses, measuring hormone levels, detecting infections and drugs, and detecting allergens.