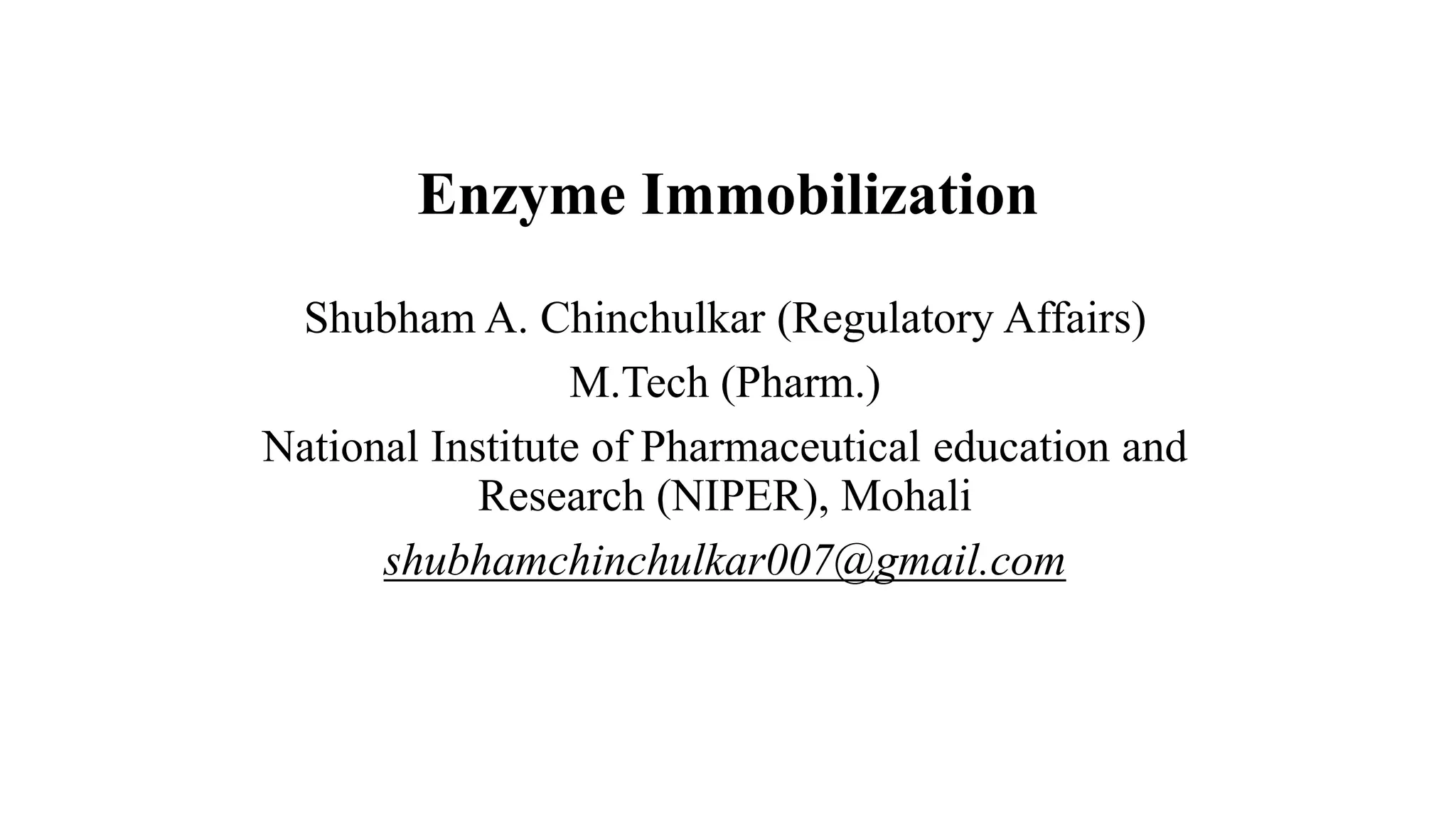
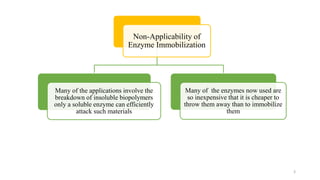
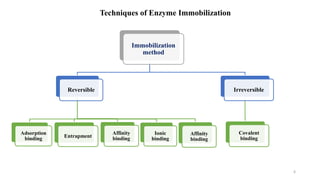
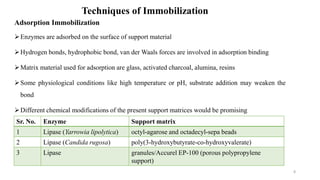
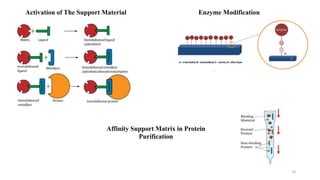
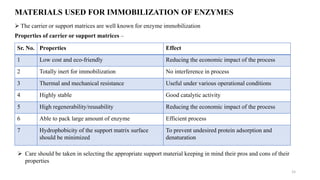
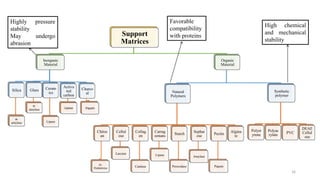
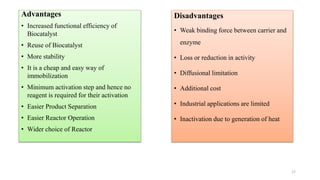
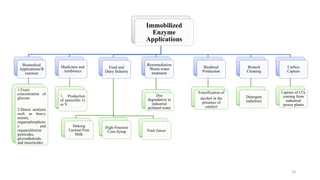
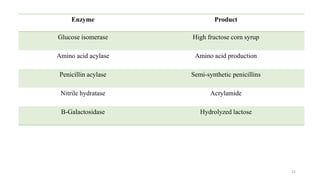
The document discusses enzyme immobilization, highlighting its historical significance and its advantages in industrial biocatalysis, including increased stability, reusability, and cost-effectiveness. It details various techniques for enzyme immobilization, such as adsorption, entrapment, and co-immobilization, along with their respective benefits and limitations. The document also explores diverse applications of immobilized enzymes in biomedicine, food production, and environmental remediation.