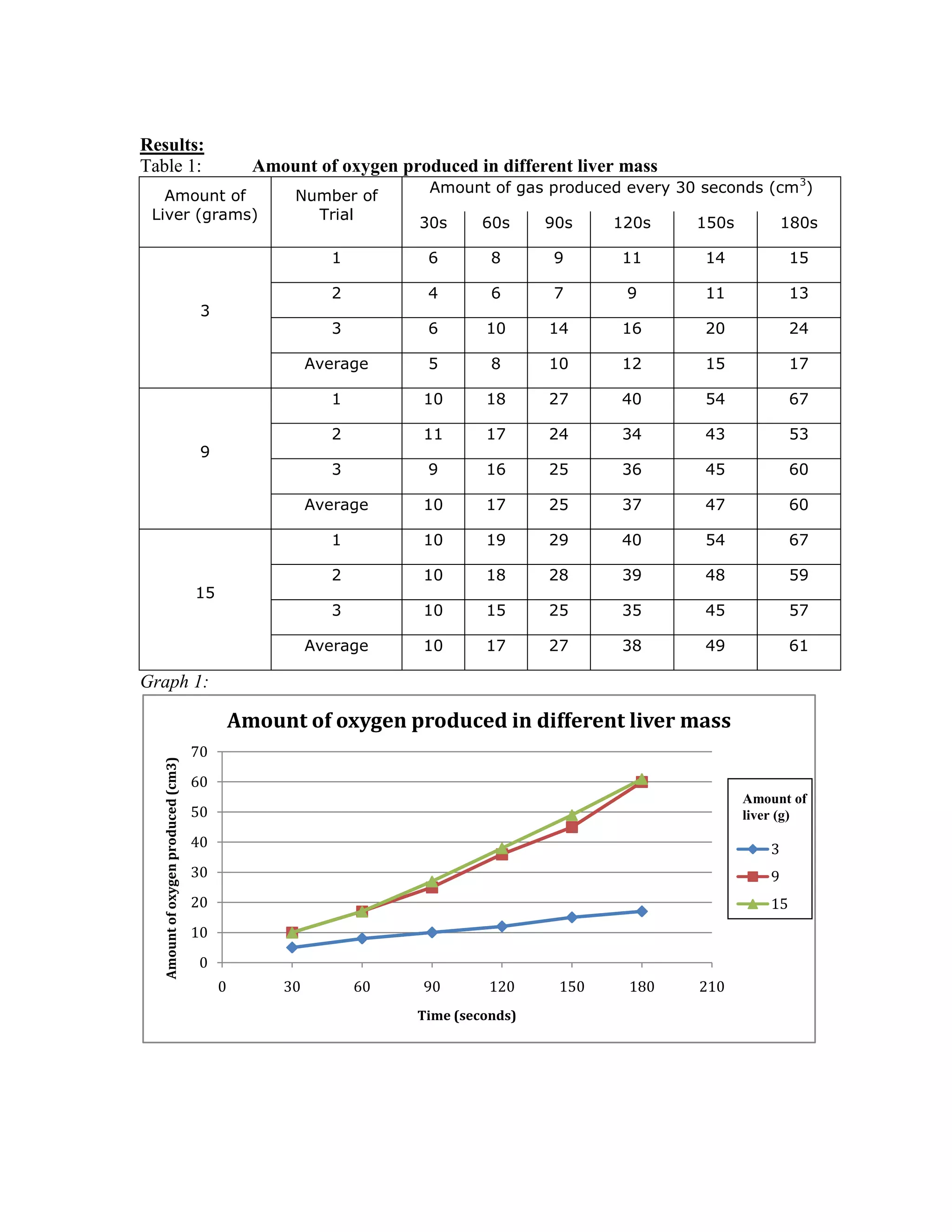
This document describes a student experiment to test the effect of varying amounts of liver on the rate of an enzymatic reaction between catalase in liver and hydrogen peroxide. The student hypothesized that increasing the amount of liver from 3g to 9g to 15g would increase the amount of oxygen produced in the reaction over time. The results showed a clear increase from 3g to 9g liver, but little difference between 9g and 15g liver. The student concluded the hypothesis was somewhat correct but noted issues with the measurement method affecting reliability of the conclusions.