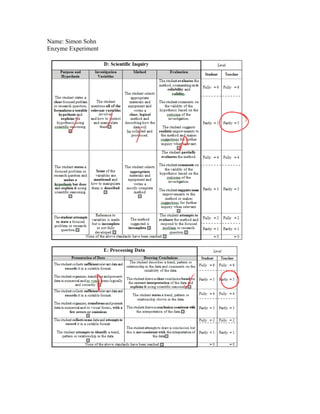
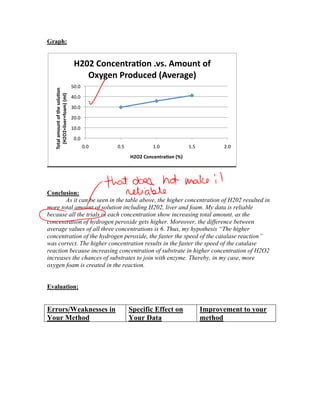
Simon Sohn conducted an experiment to test the effect of hydrogen peroxide concentration on the rate of a catalase reaction. He hypothesized that a higher hydrogen peroxide concentration would result in a faster reaction rate and more oxygen production. The experiment involved measuring oxygen production over 1 minute for 3 concentrations of hydrogen peroxide (0.5%, 1%, 1.5%) using a constant amount of liver. The results supported the hypothesis, showing more oxygen produced with increasing concentration. However, the experiment could be improved by standardizing the liver sample size and ensuring it fully submerged in the hydrogen peroxide.