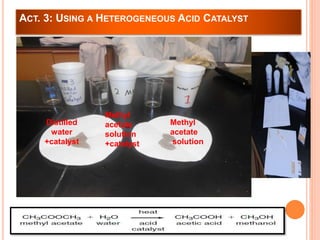
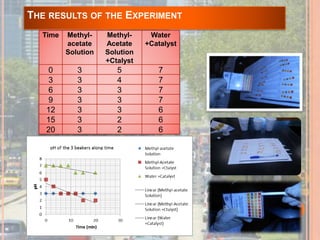
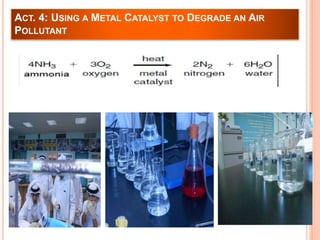
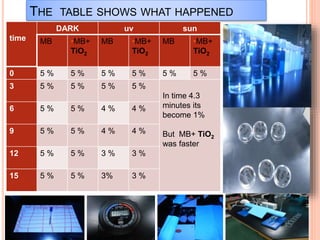
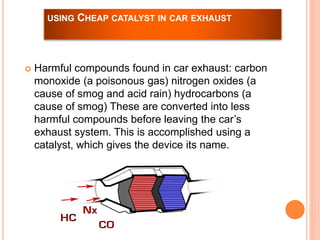
The document outlines a series of activities related to environmental catalysis, with a focus on using low-cost catalysts to mitigate pollutants in car exhaust. It discusses various types of catalysts, their importance in chemical reactions, and the effectiveness of cheaper alternatives to precious metals in catalytic converters to reduce harmful emissions. The study highlights the significant pollution reduction achieved through catalytic converters, converting toxic gases into less harmful compounds.