The document discusses the energy required for various everyday activities and products, including:
- Lighting a 100W bulb for 1 year requires 326kg of coal and emits 750kg of CO2.
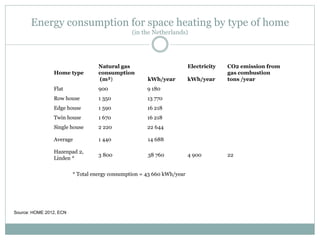
- Heating 1000L of water from 20-100°C requires about 1/10 the energy of lighting a 100W bulb for 1 year.
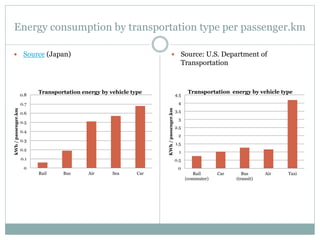
- Transportation by car is less energy efficient than other modes like rail or bus.
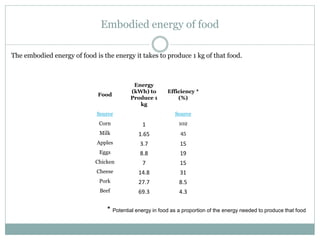
- The embodied energy to produce an average car is about 5 times more than the energy used to drive it 20,000km per year.
![An example of what it means to let a lightbulb of 100 W emit
light continuously for 1 year
We assume electricity generation is by a coal power
plant. The energy density of coal is roughly 6.7 kWh/kg
(kWh = kilowatt-hour) . This corresponds to 0.765 Wy
(Watt-year. Conversion of coal to electricity has an
efficiency of 40%. So, 1 kg coal can generate 0.4 x 0.765
= 0.306 W for 1 year. Inversely, to generate 100 W for 1
year, we need 326 kg of coal. [79]
(Note that 100 Wy = 876 kWh)
How much CO2 is emitted during that time?
CO2 emission from coal = 2.3 kg/kg coal
(anthracite)
So, 326 kg coal emits 750 kg CO2 in the atmosphere
From EIA](https://image.slidesharecdn.com/energyineverydaylife-140702124106-phpapp01/75/Energy-in-everyday-life-1-2048.jpg)


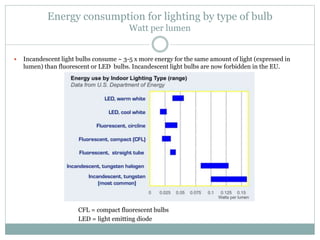
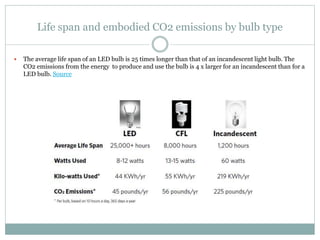
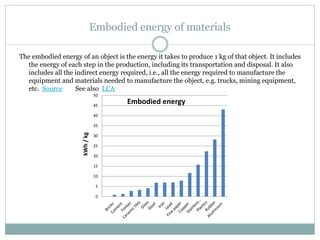
![Embodied energy of a car
Treloar, et al. have estimated the embodied energy in an average automobile in Australia as
270 GJ (gigajoules) (= 75 000 kWh) with a life span of 15 years for the car. Note that the
CO2 emission to make a car = 16 tons (0.27 kg/kWh). For all cars in the world (~1
billion) that would be 16 gigatons [Ref]erence] .
A similar calculation is based on the Toyota Prius, an energy efficient car on the road.
Embodied energy is 165 GJ, half of which is in steel and aluminium. This is 40 % lower
than the average Australian car (from: click here).
1 GJ= 31.71 x 10-12 TWy = 277 780 x 10-12 TWh = 277 780 x 10-3 kWh = 278 kWh 1MJ = 0.278 kWh
From wattzon.com](https://image.slidesharecdn.com/energyineverydaylife-140702124106-phpapp01/85/Energy-in-everyday-life-9-320.jpg)