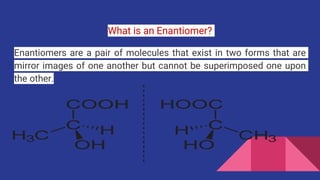
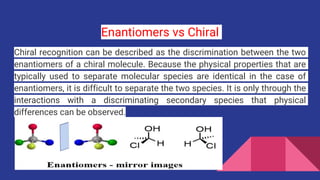
Enantiomers are a pair of molecules that are non-superimposable mirror images of each other. They are chemically identical but rotate polarized light in opposite directions. A mixture with equal proportions of both enantiomers is called a racemic mixture and does not rotate polarized light. Enantiomers exist when a carbon atom is bonded to four different groups. They have identical physical properties except for how they interact with polarized light.