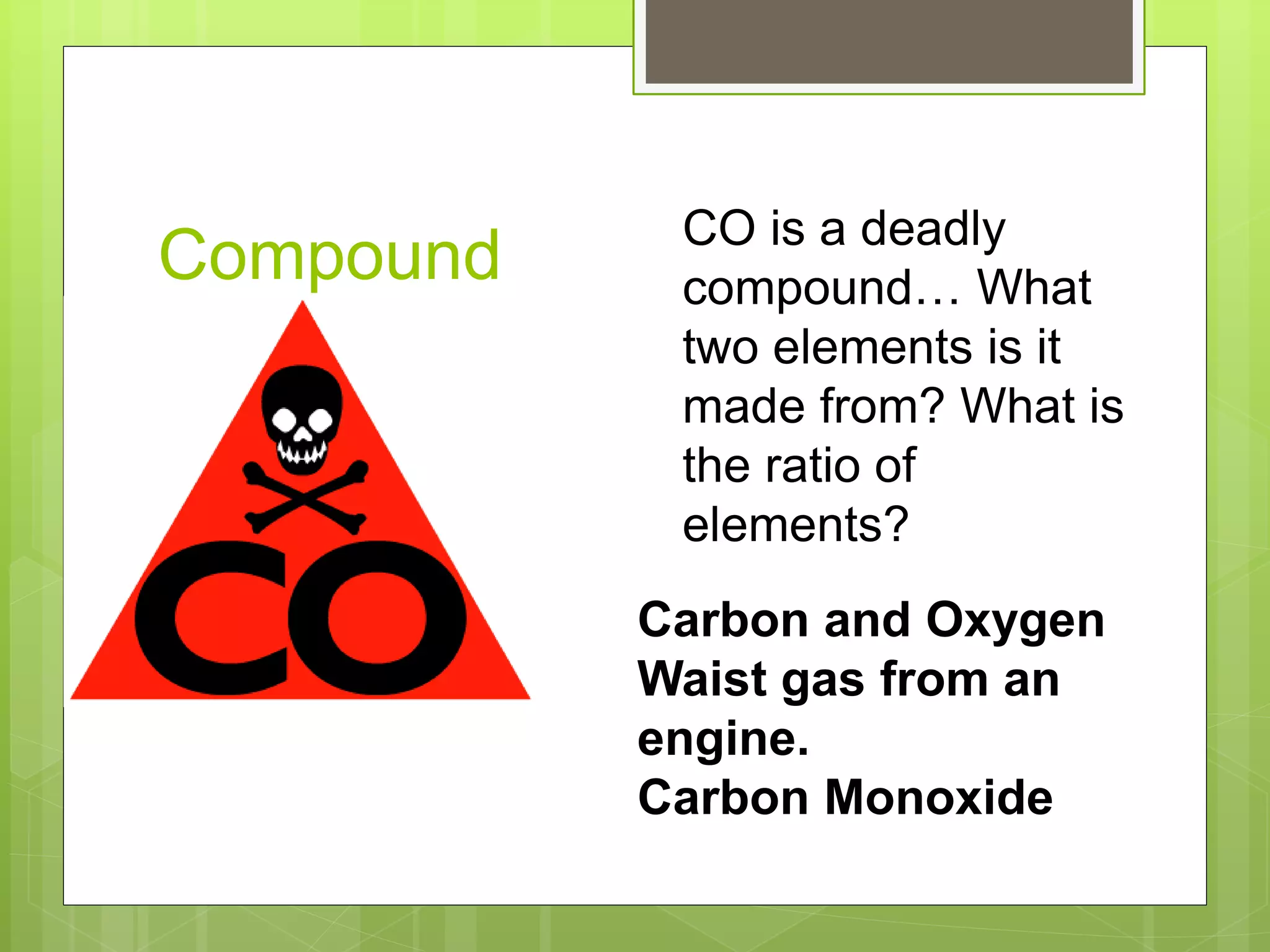
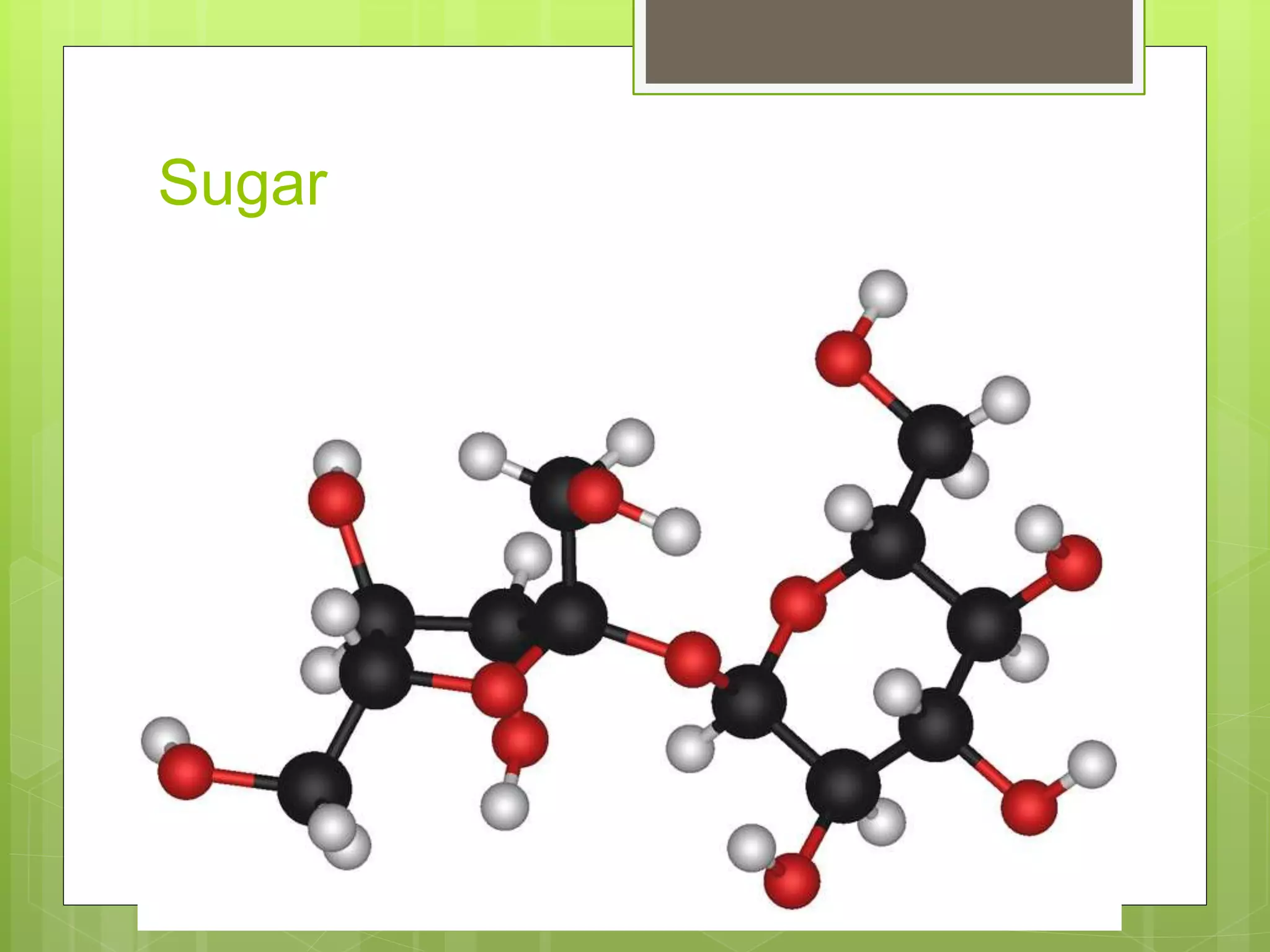
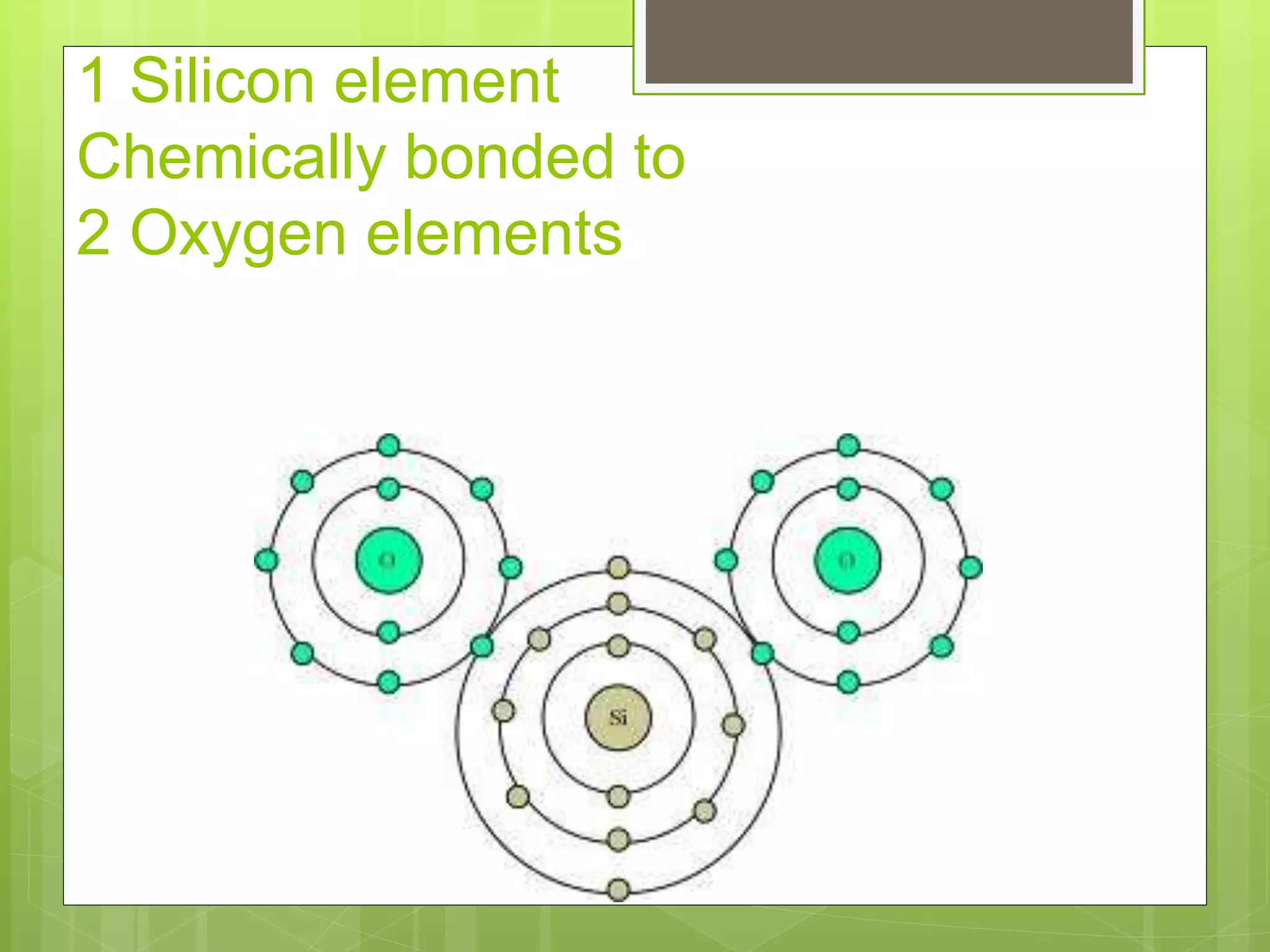
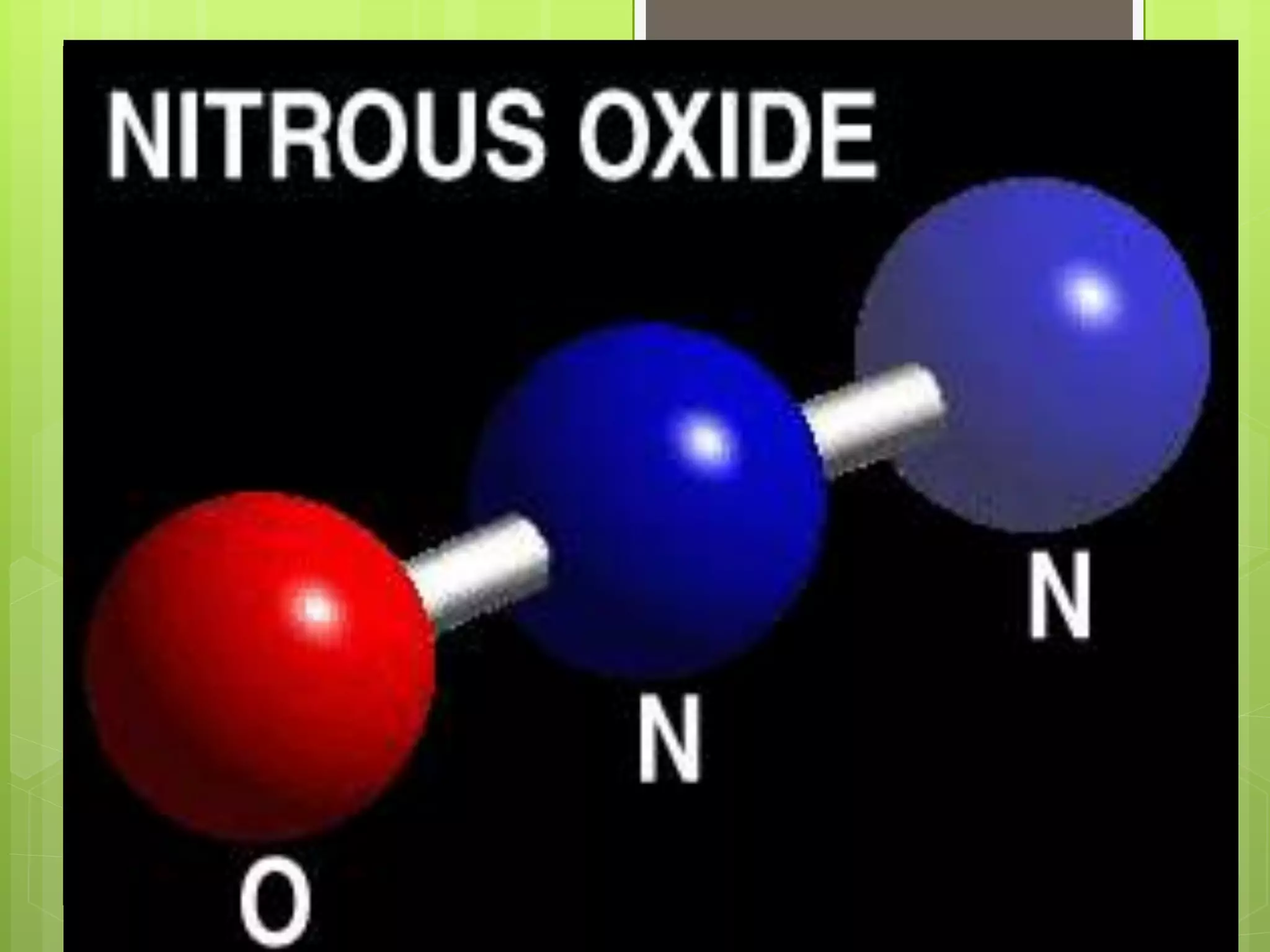
The document provides information to distinguish between elements and compounds. It notes that elements are pure substances made of only one type of atom, while compounds contain two or more different types of atoms bonded together in a specific ratio. Several examples of common elements and compounds are given, along with their constituent elements and ratios. The last part describes a hypothetical scenario in 2032 where the reader discovers a new compound on Jupiter and must document its name, properties, constituent elements, and atomic structure.