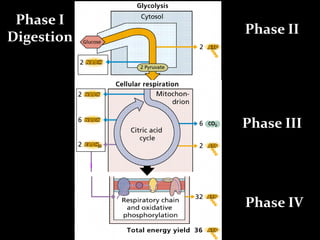
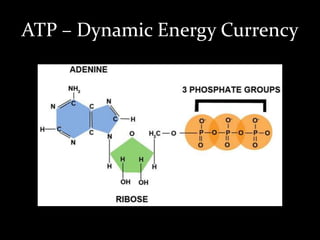
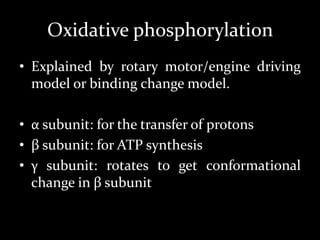
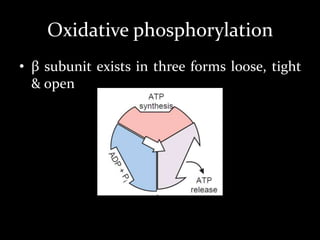
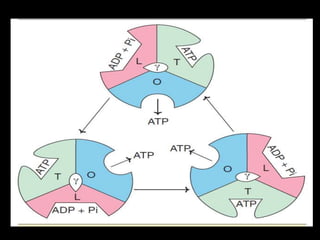
1. The document discusses cellular energetics and the electron transport chain (ETC) that generates ATP through oxidative phosphorylation.
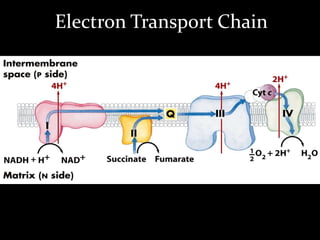
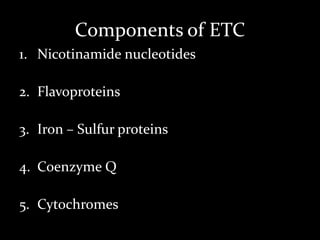
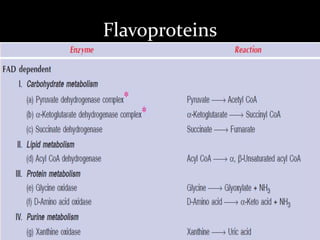
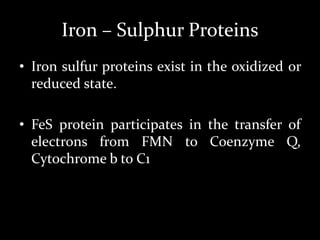
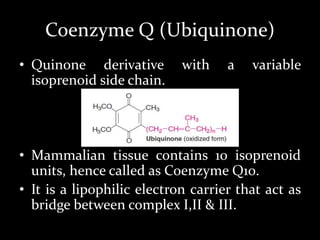
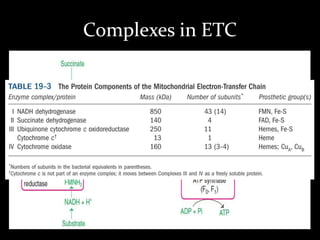
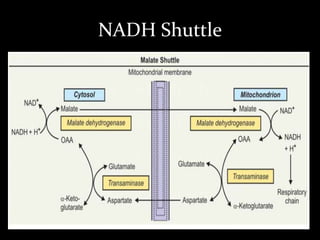
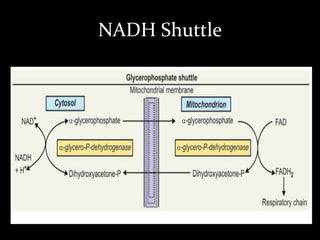
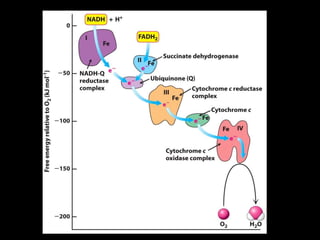
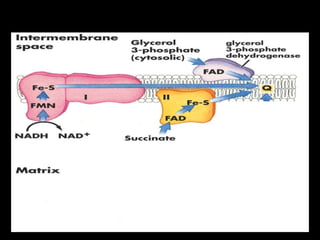
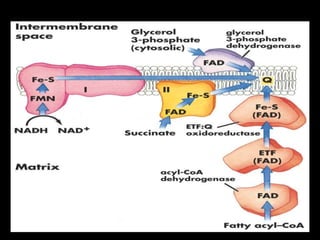
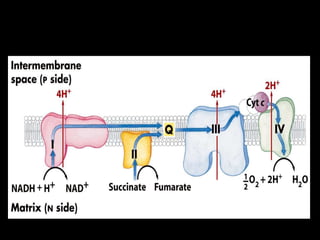
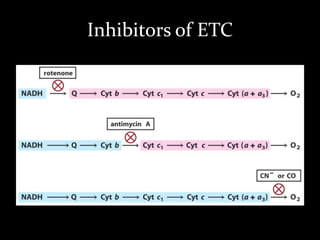
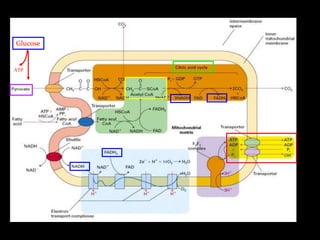
2. The ETC consists of protein complexes and electron carriers like nicotinamide nucleotides, flavoproteins, iron-sulfur proteins, coenzyme Q, and cytochromes that transfer electrons down the chain.
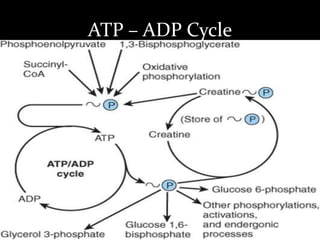
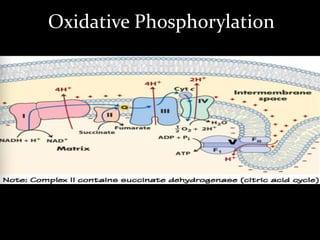
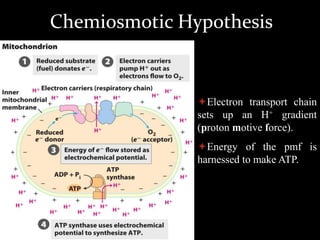
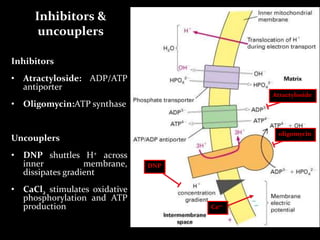
3. As electrons are passed down the ETC, protons are pumped from the mitochondrial matrix into the intermembrane space, generating a proton gradient. ATP synthase harnesses the potential energy of this proton gradient to drive the phosphorylation of ADP to ATP.