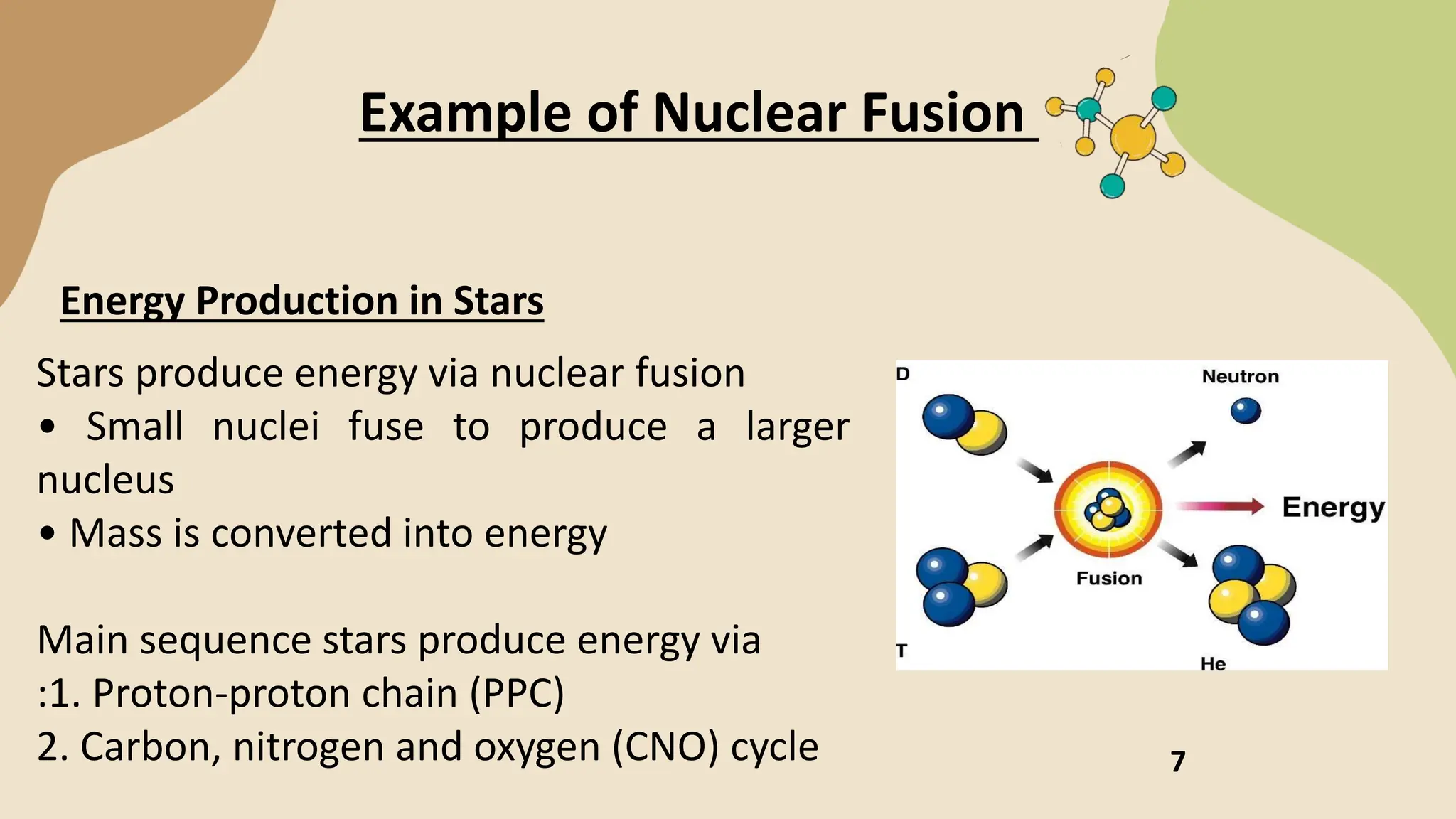
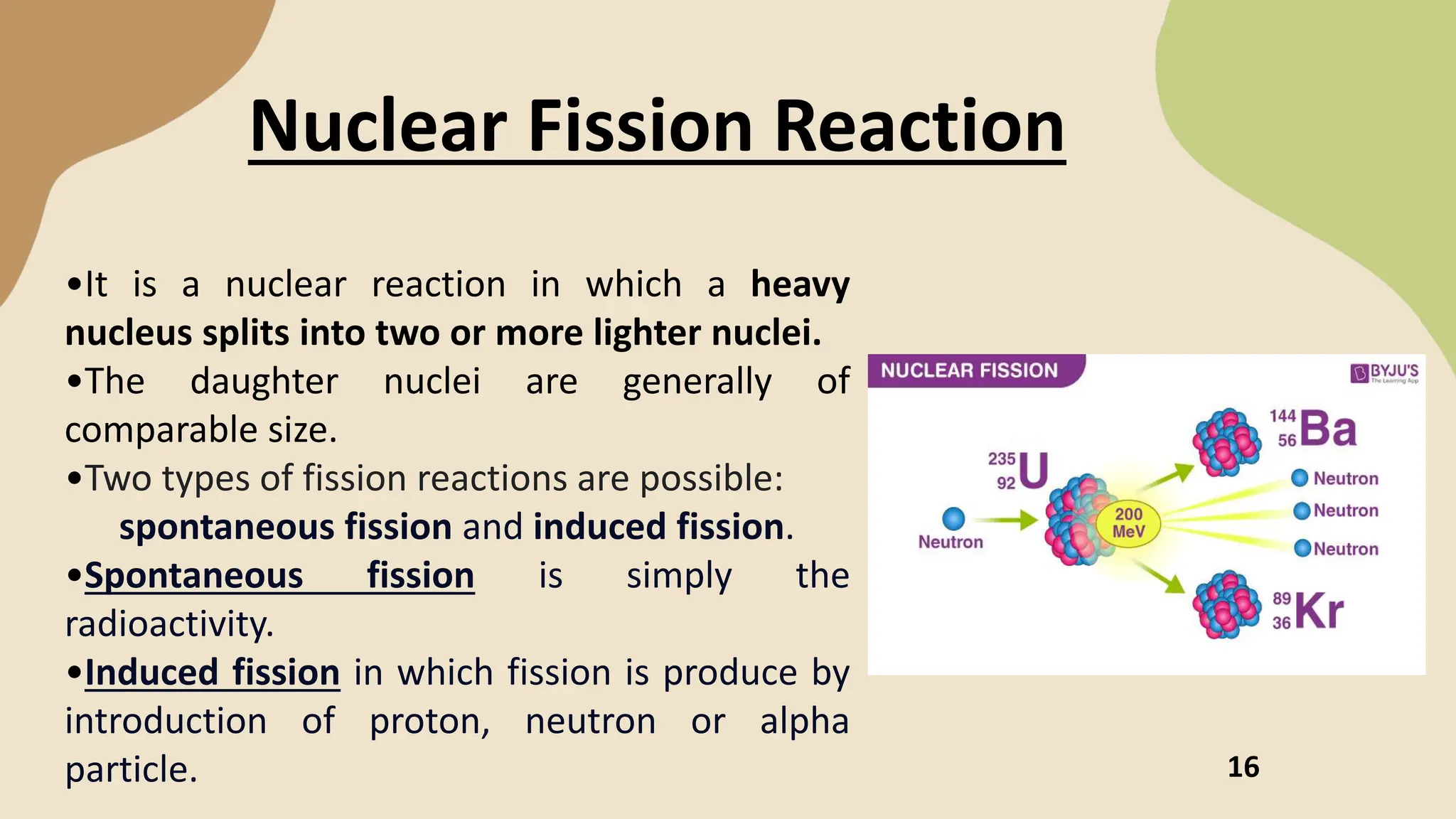
The document discusses nuclear fission and fusion, highlighting their processes, energy production mechanisms, and challenges in harnessing these reactions. Fission entails the splitting of a heavy nucleus into lighter ones, while fusion combines light nuclei to create heavier ones, both releasing energy under certain conditions. It emphasizes advancements in fusion technology and the potential of nuclear energy as a clean alternative to fossil fuels.




![Fusion Reaction Nuclear Binding Energy
Δm = [ZmH + (A-Z) mn]-M
•Greater the binding energy greater will
be the stability
6](https://image.slidesharecdn.com/fissionandfusionreaction-240530155529-c8e5be99/75/Fission-and-fusion-reaction-presentation-pptx-6-2048.jpg)