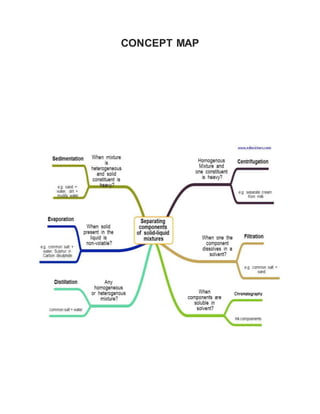
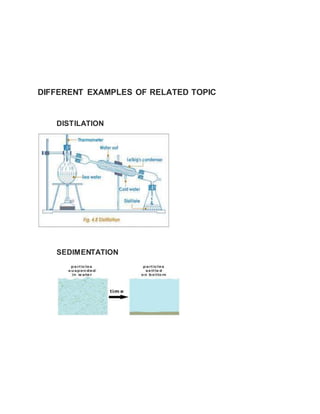
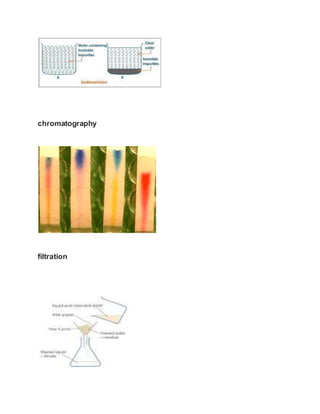
This document discusses the development of e-content on the topic of separating mixtures. It aims to explain the concept of different types of mixtures and techniques for their separation. These techniques include distillation, chromatography, sedimentation, filtration, decantation and evaporation. Distillation separates mixtures by selective vaporization and condensation. Chromatography separates mixtures based on differences in solubility and adsorption. Sedimentation, filtration and decantation separate mixtures based on differences in properties like size, shape and density. Overall, the document conveys that separation techniques exploit differences in chemical or physical properties between mixture components.