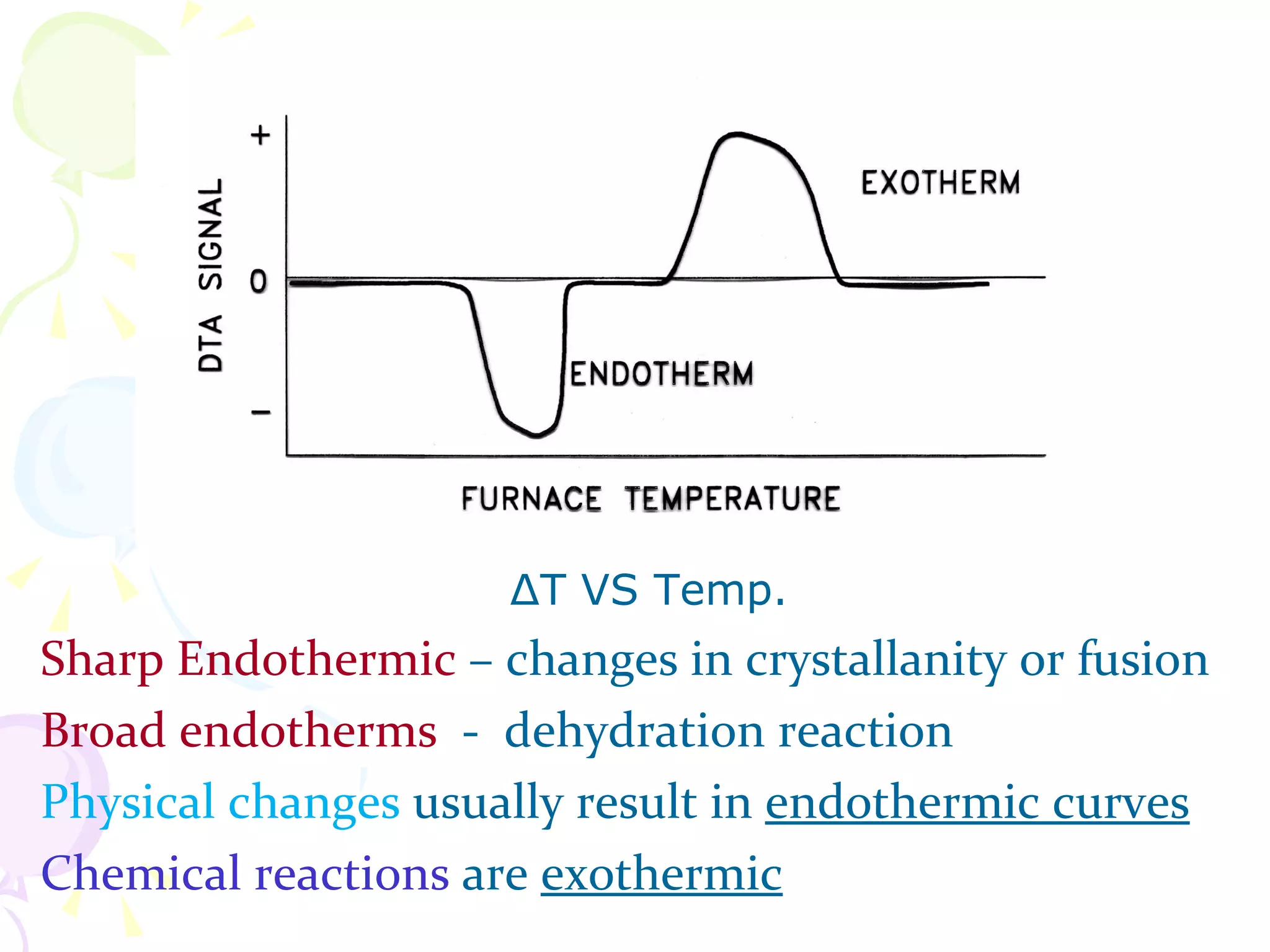
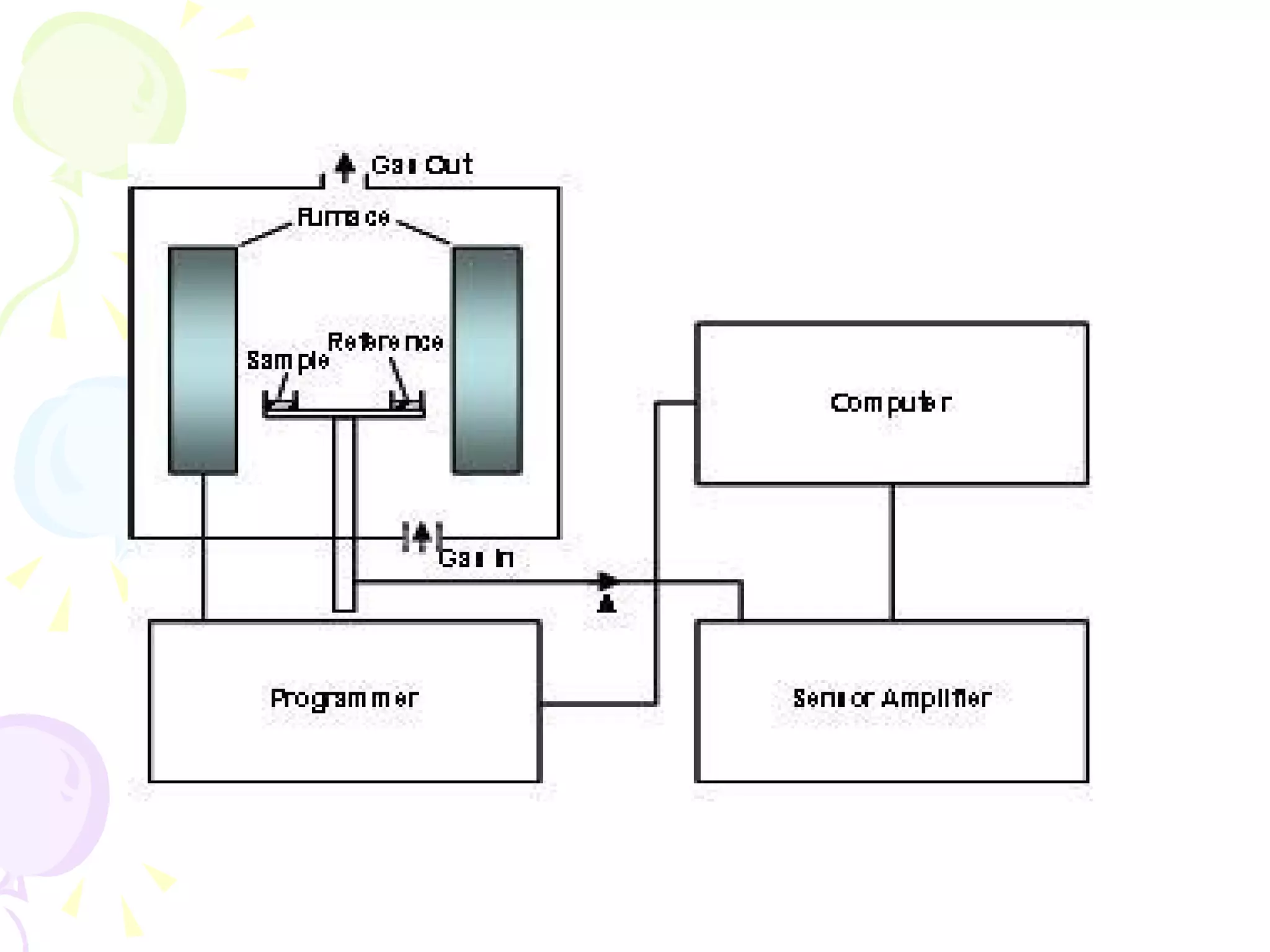
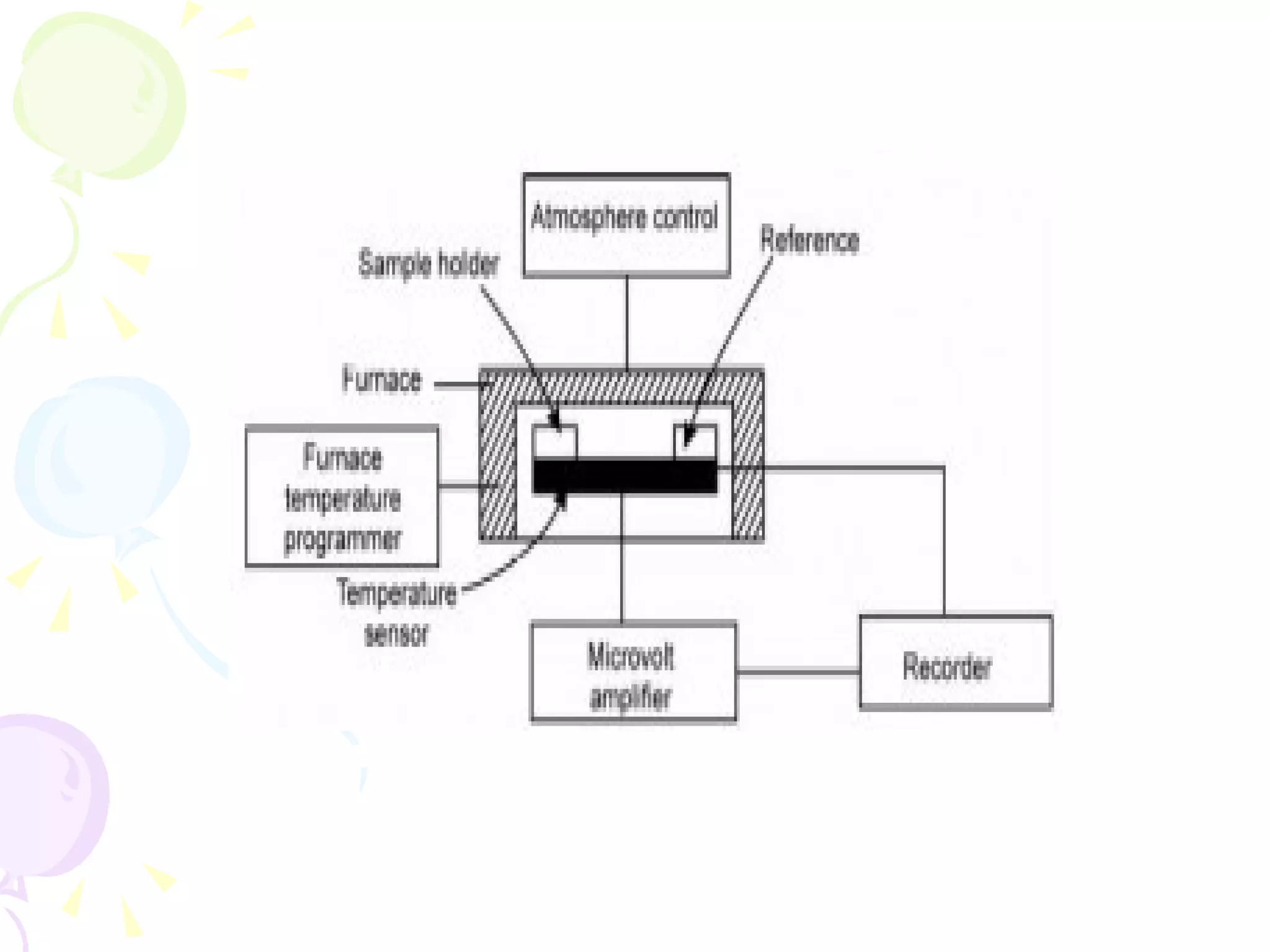
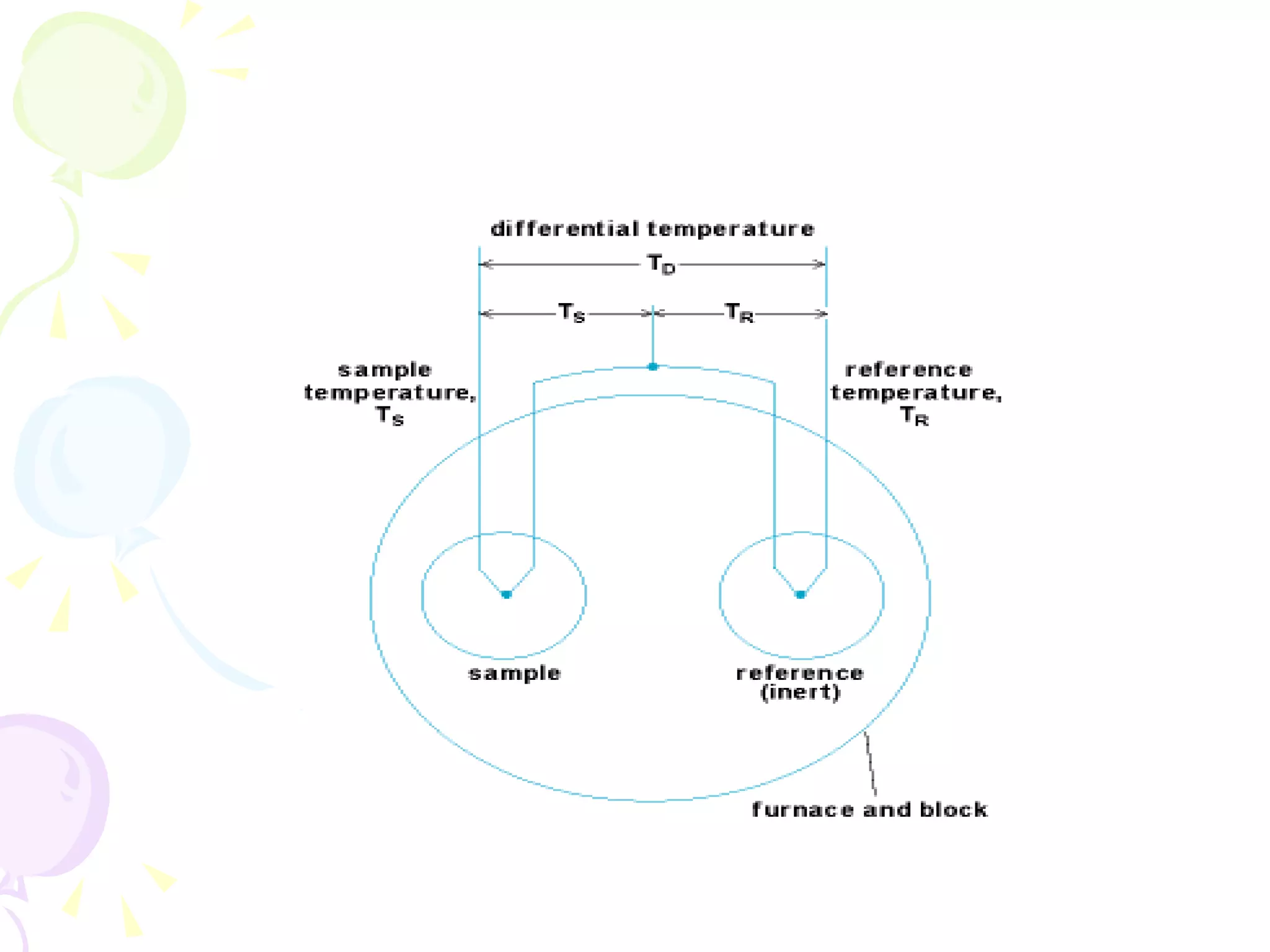
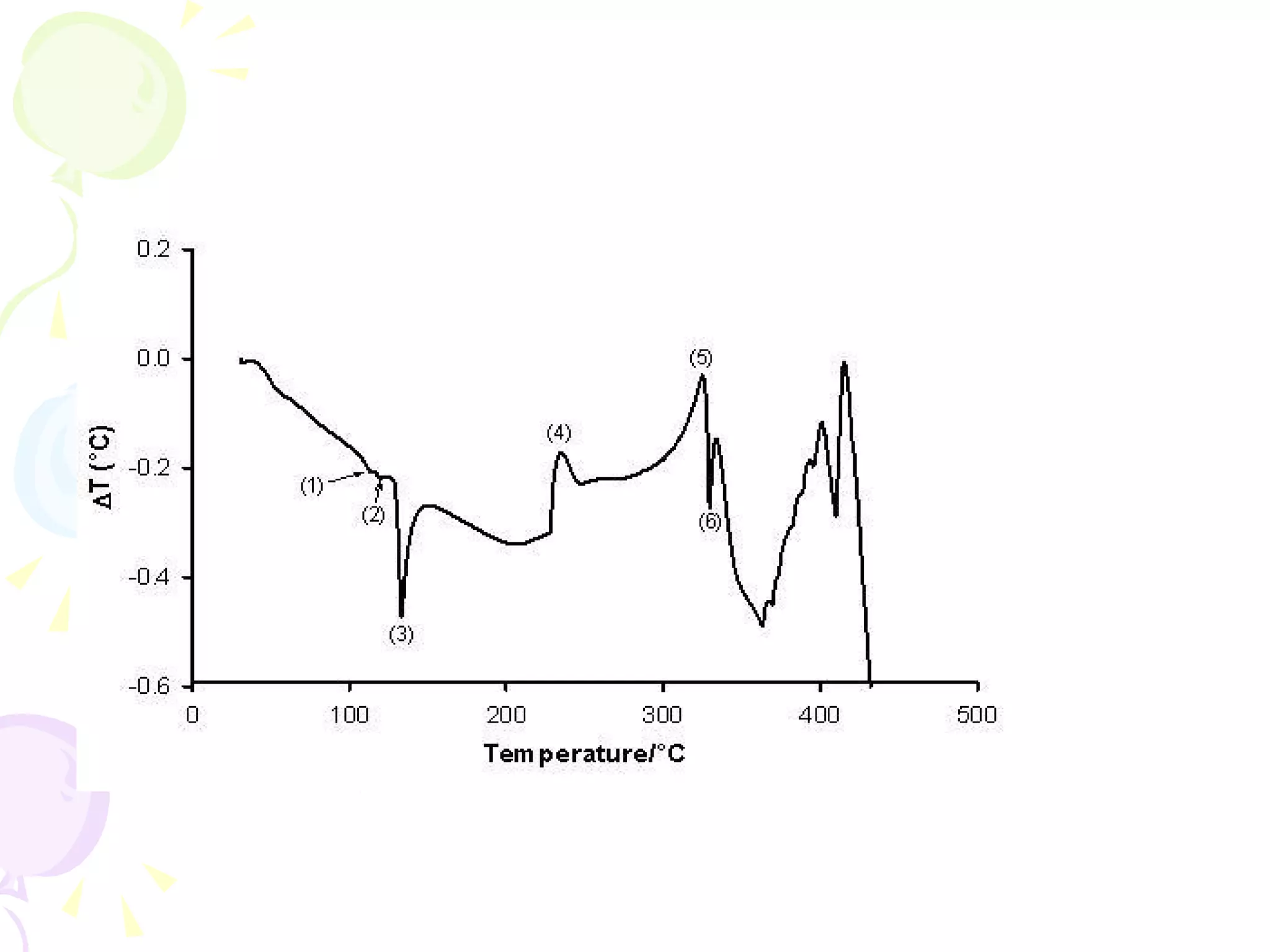
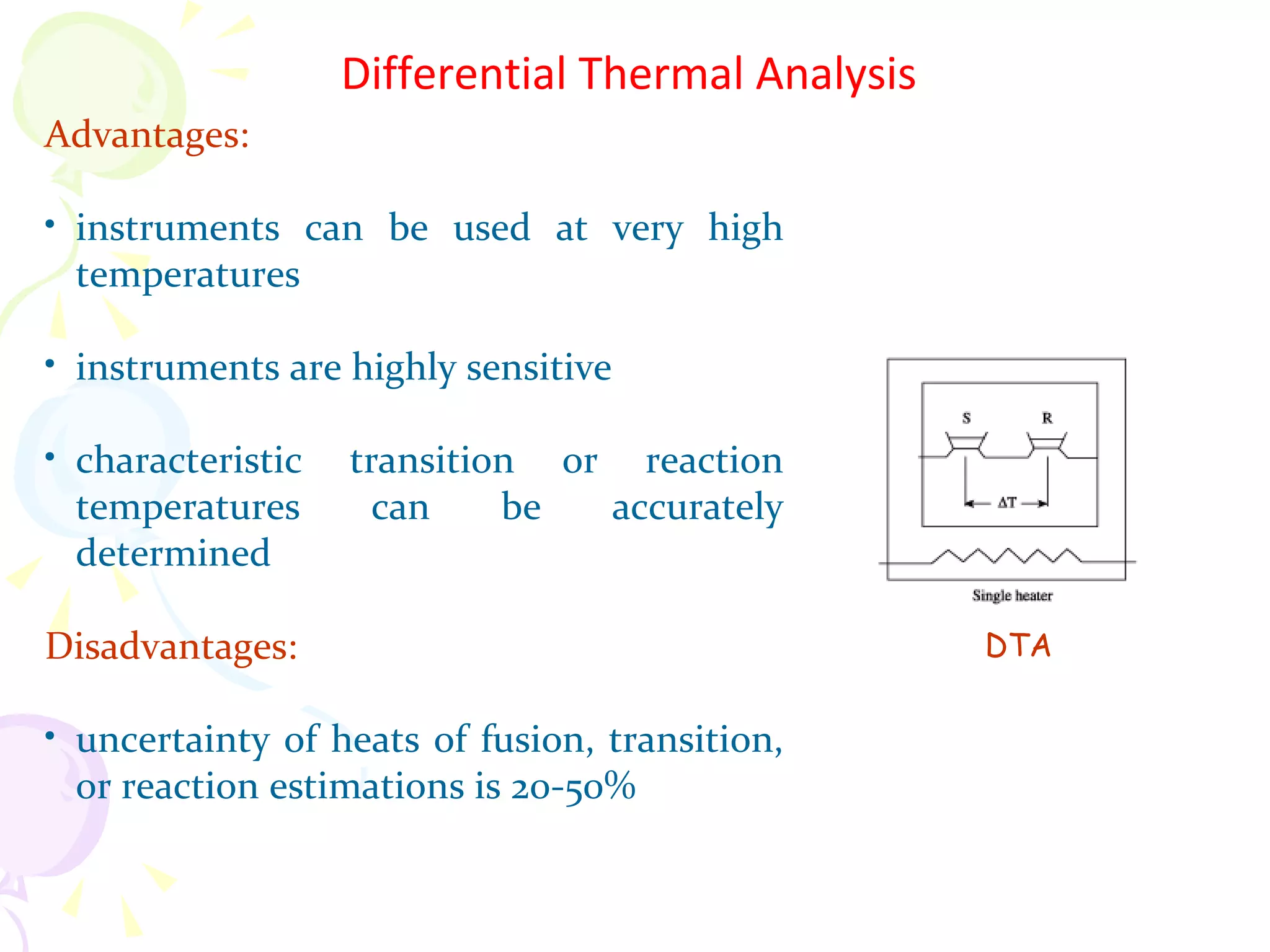
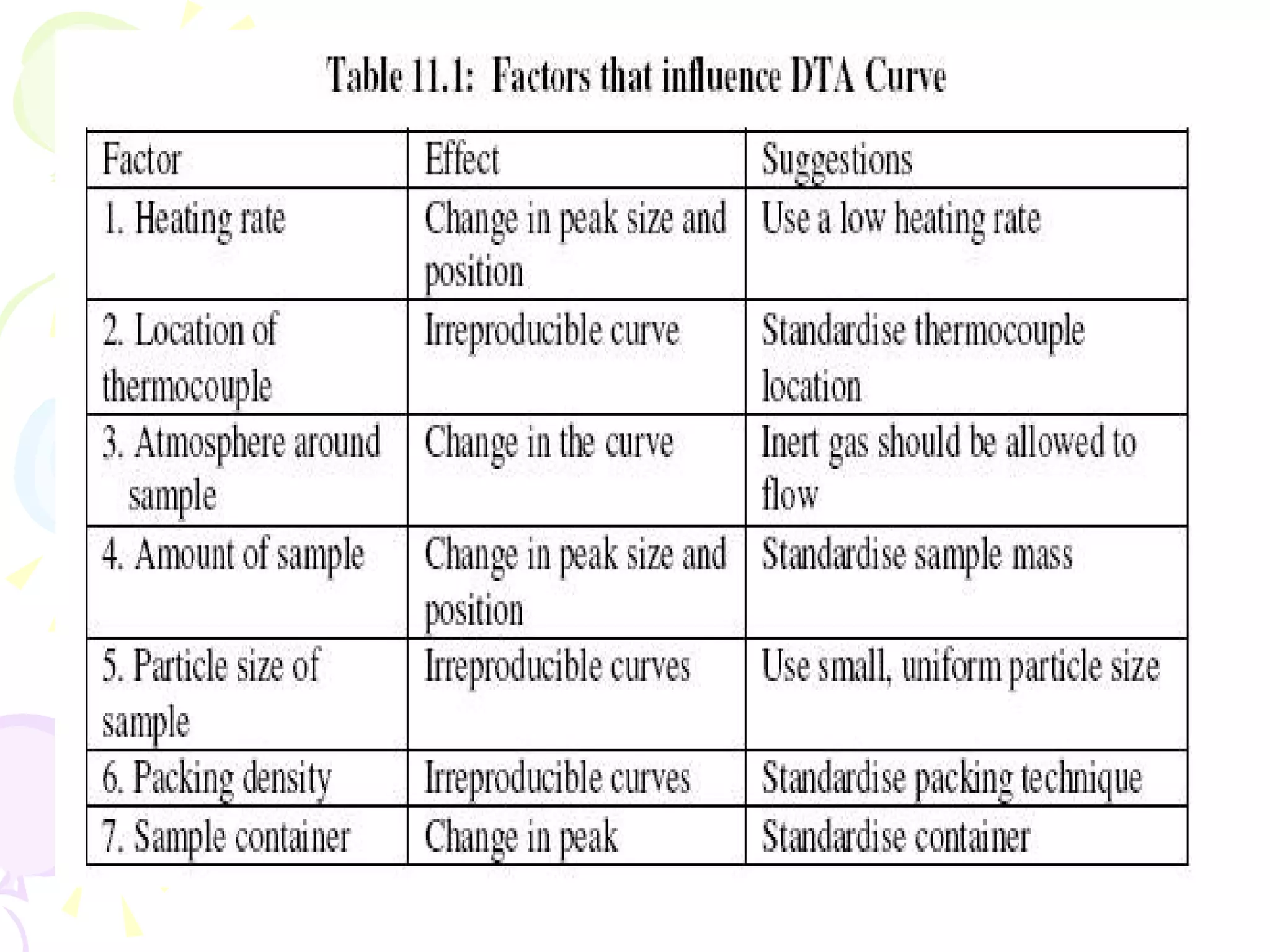
Differential thermal analysis (DTA) is a thermal analysis technique that measures the temperature difference between a sample and an inert reference material as they are heated or cooled under identical conditions. [DTA] curves provide information about physical and chemical changes in a material as a function of temperature or time, such as fusion, decomposition, or phase transitions. The DTA technique involves heating a sample and reference material simultaneously while measuring any temperature differences between the two. Changes in the sample, such as exothermic or endothermic reactions, will result in temperature differences compared to the inert reference curve. DTA can be used to identify materials and assess purity by comparing sample curves to reference curves.