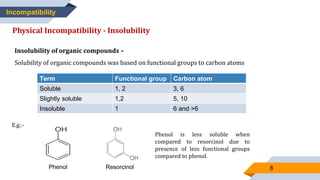
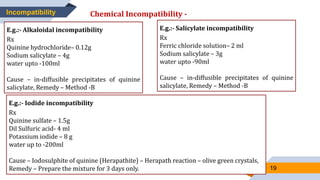
The document discusses incompatibilities in pharmacy prescriptions, categorizing them into physical, chemical, and therapeutic types. It elaborates on physical incompatibilities such as insolubility, immiscibility, liquefaction, and precipitation, as well as chemical incompatibilities that lead to changes in drug properties. It also addresses therapeutic incompatibilities that arise from errors in dosage, contraindicated drugs, and drug interactions.