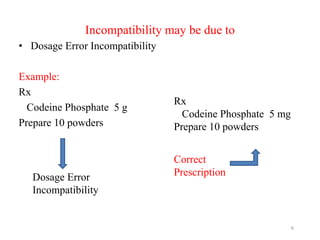
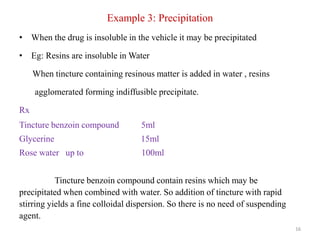
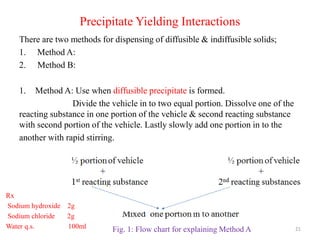
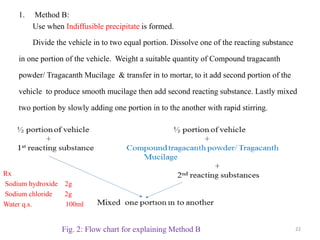
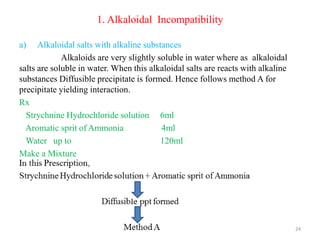
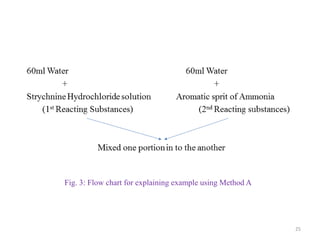
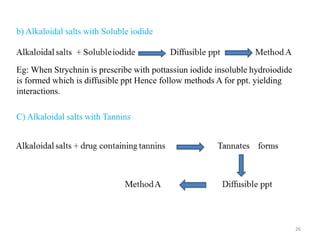
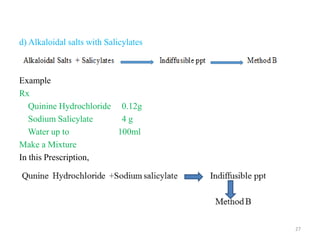
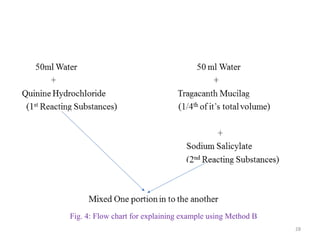
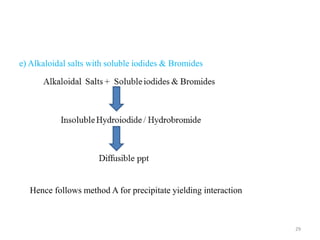
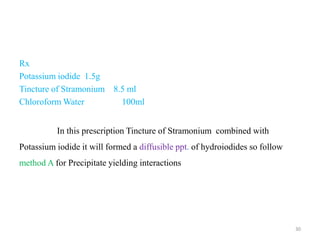
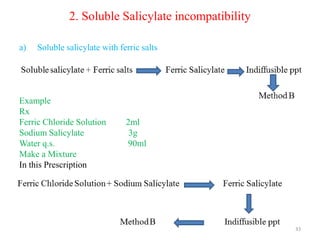
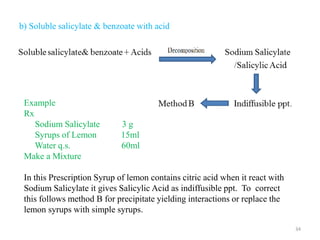
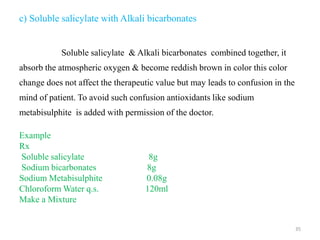
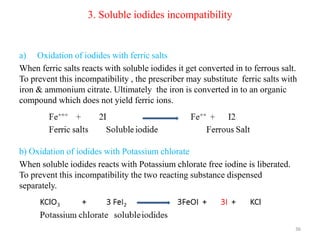
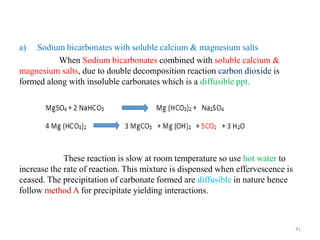
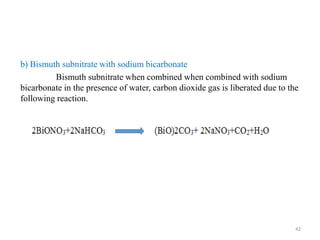
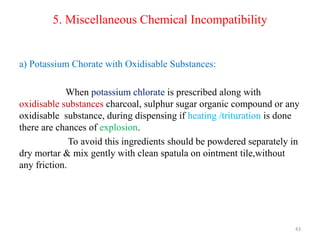
The document provides an overview of drug incompatibility, which is defined as the undesirable effects resulting from mixing incompatible substances, impacting the safety and efficacy of pharmaceutical dosage forms. It categorizes incompatibilities into physical, chemical, and therapeutic types, with detailed examples and methods for correction. Emphasis is placed on identifying and addressing issues like dosage errors, mixture incompatibility, and the need for caution in prescription practices.