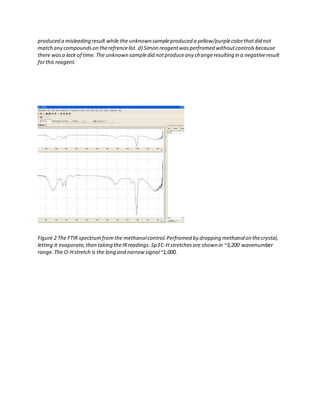
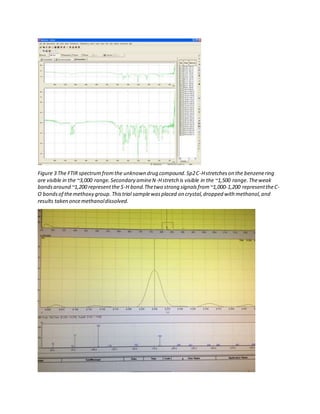
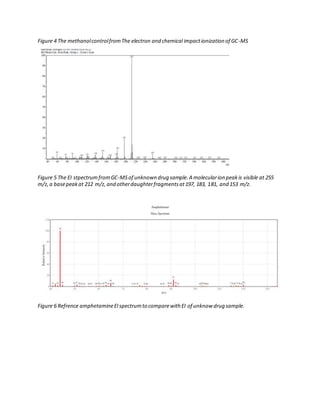
The document details the analysis of an unknown 1 mg white powder drug sample using various presumptive tests, Fourier-transform infrared spectroscopy (FTIR), and gas chromatography-mass spectrometry (GC-MS). Presumptive tests including the Marquis, Mecke, Mandelin, and Simon reagents indicated the sample was similar to 2C-T-7. FTIR analysis found secondary amine and methoxy group functionalities. GC-MS analysis under electron ionization produced a molecular ion peak at 255 m/z and daughter fragments consistent with a 2C-T derivative. Chemical ionization produced a similar spectrum, confirming the identification.