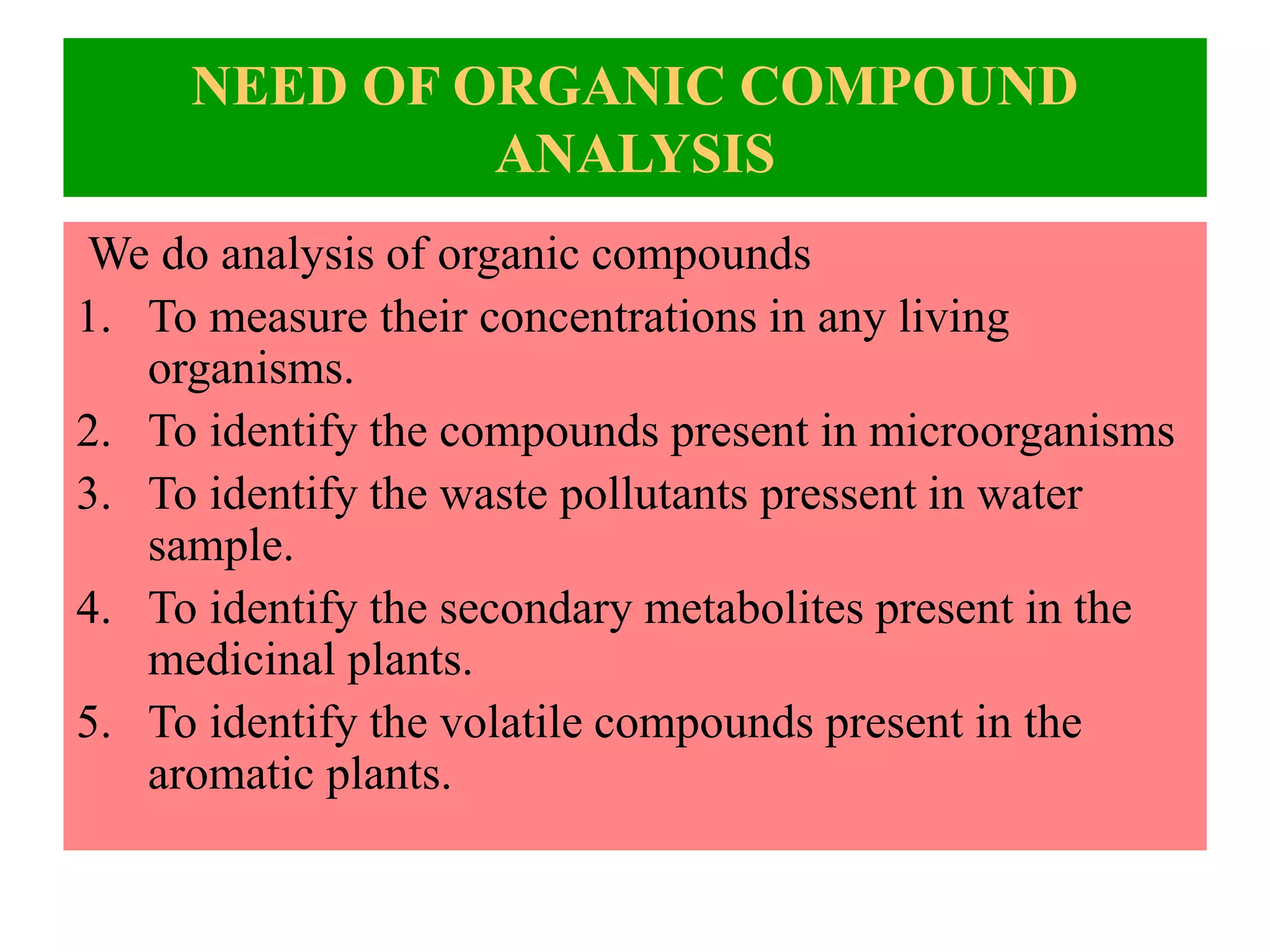
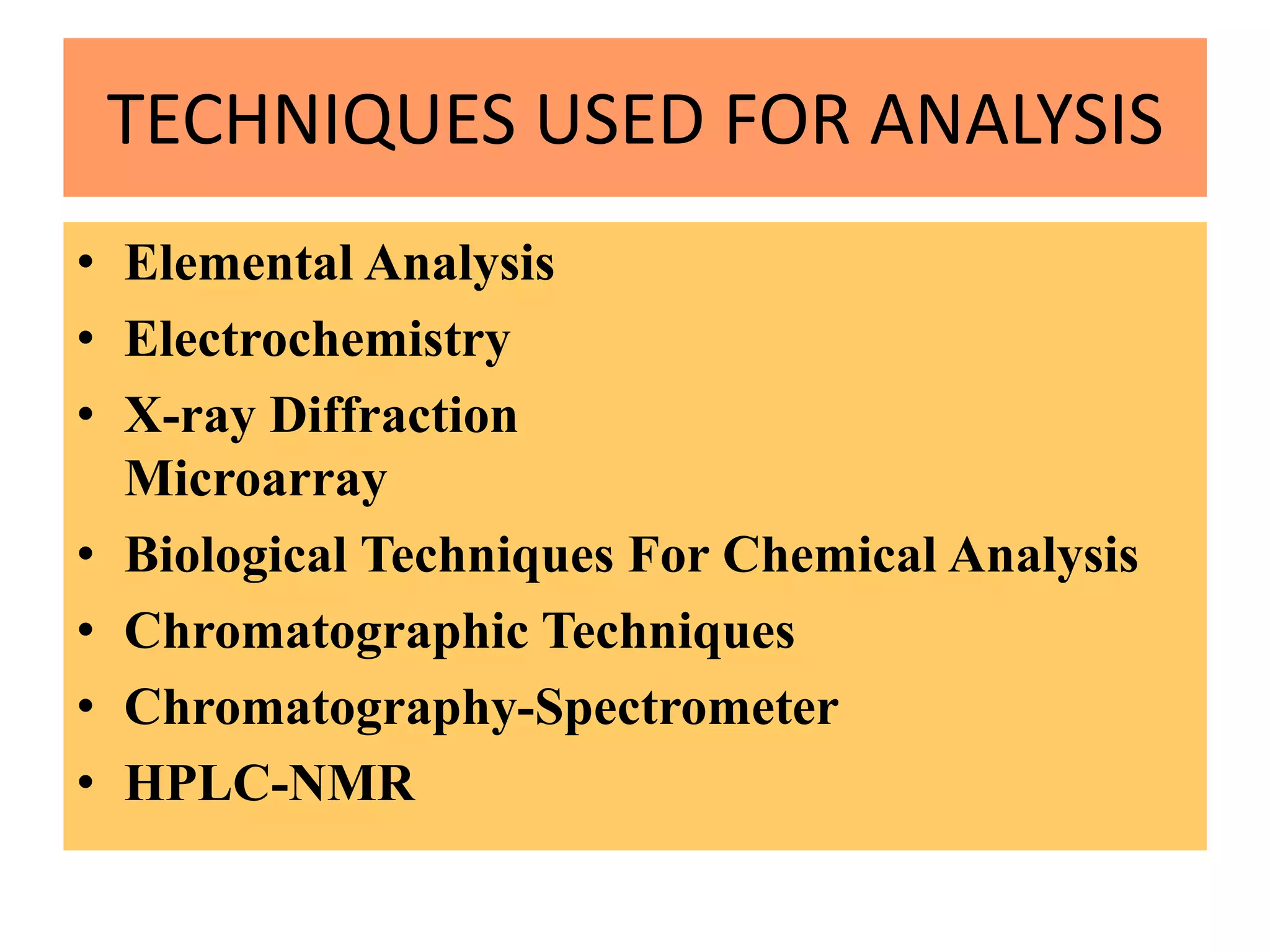
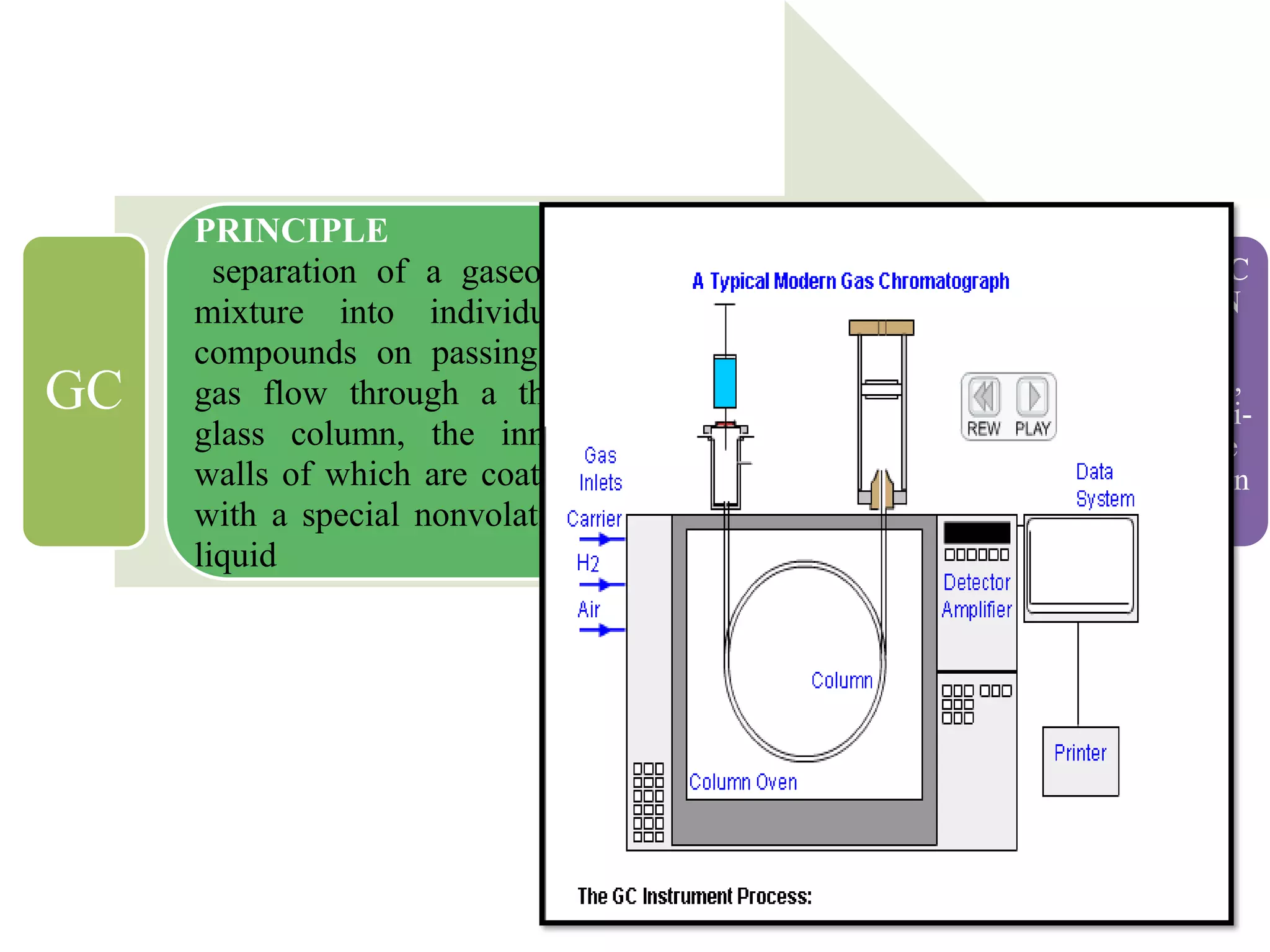
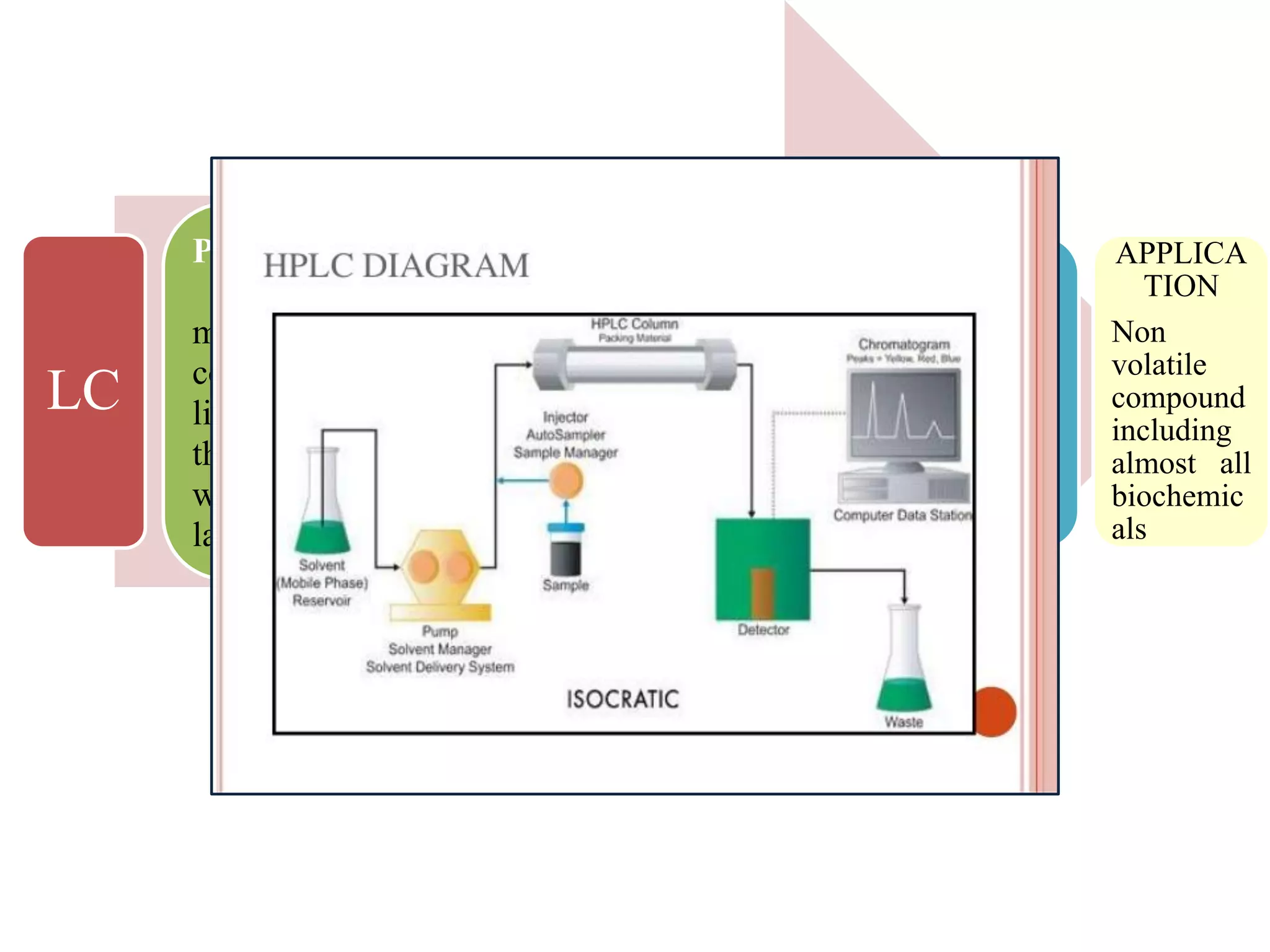
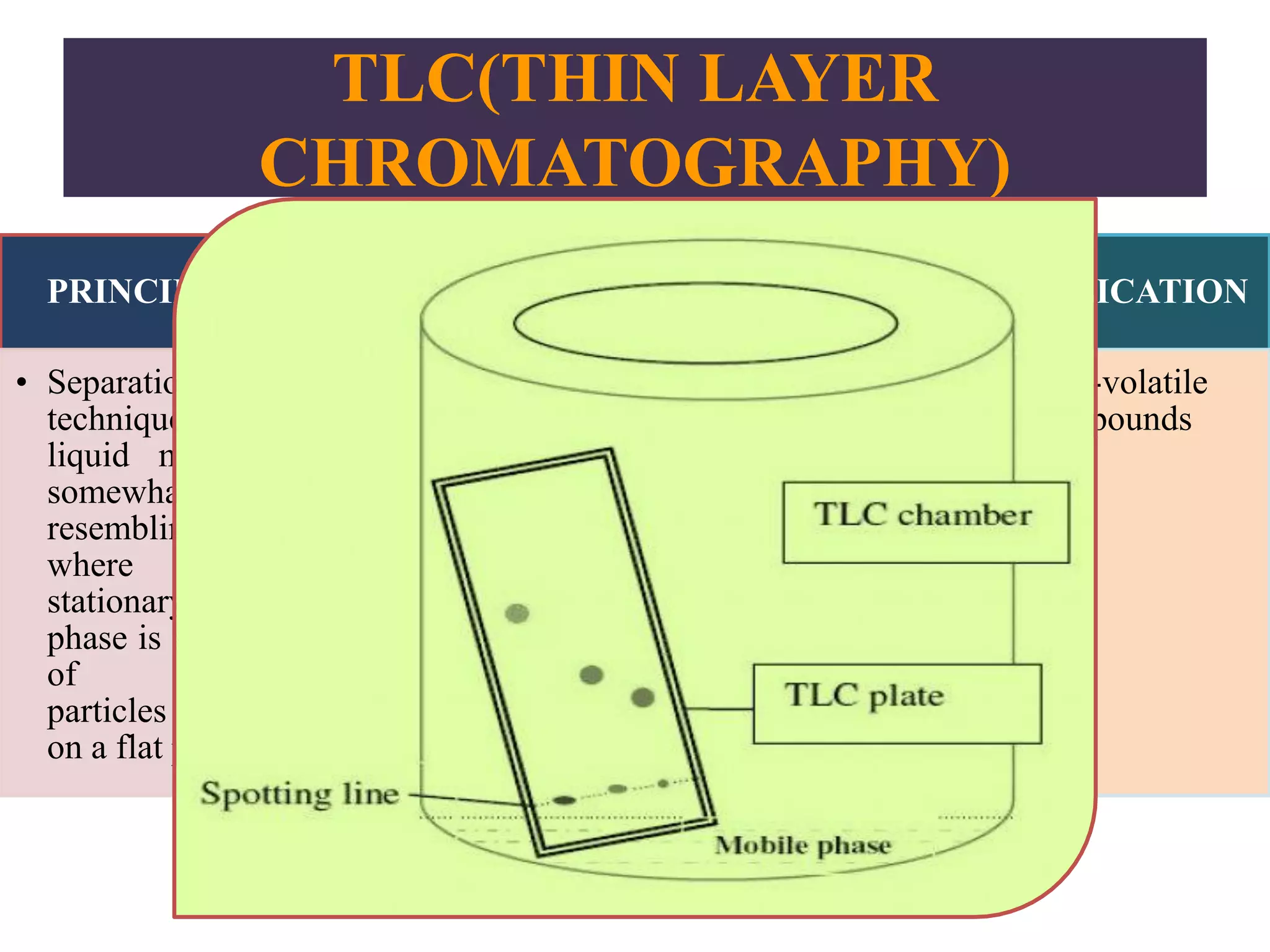
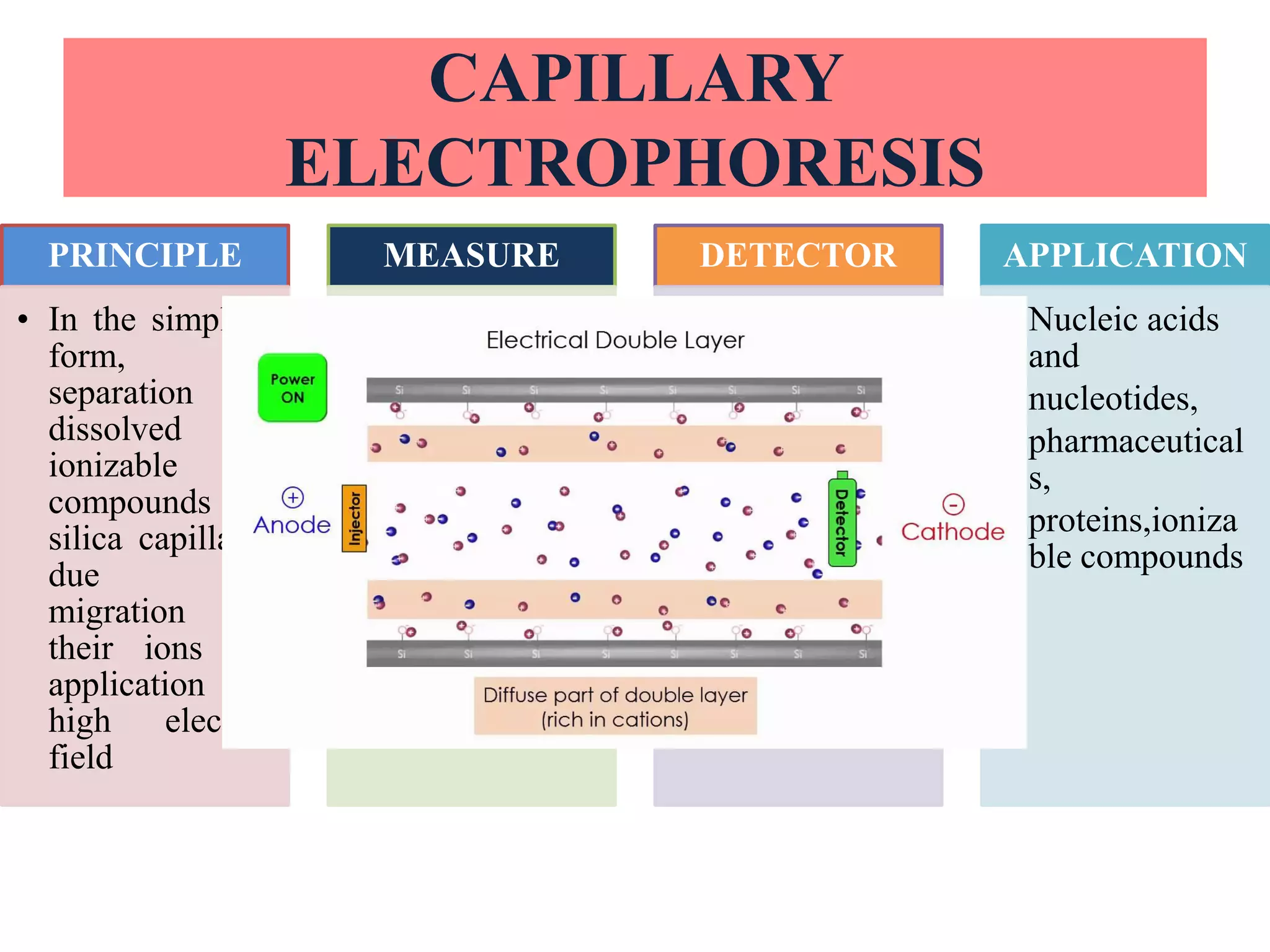
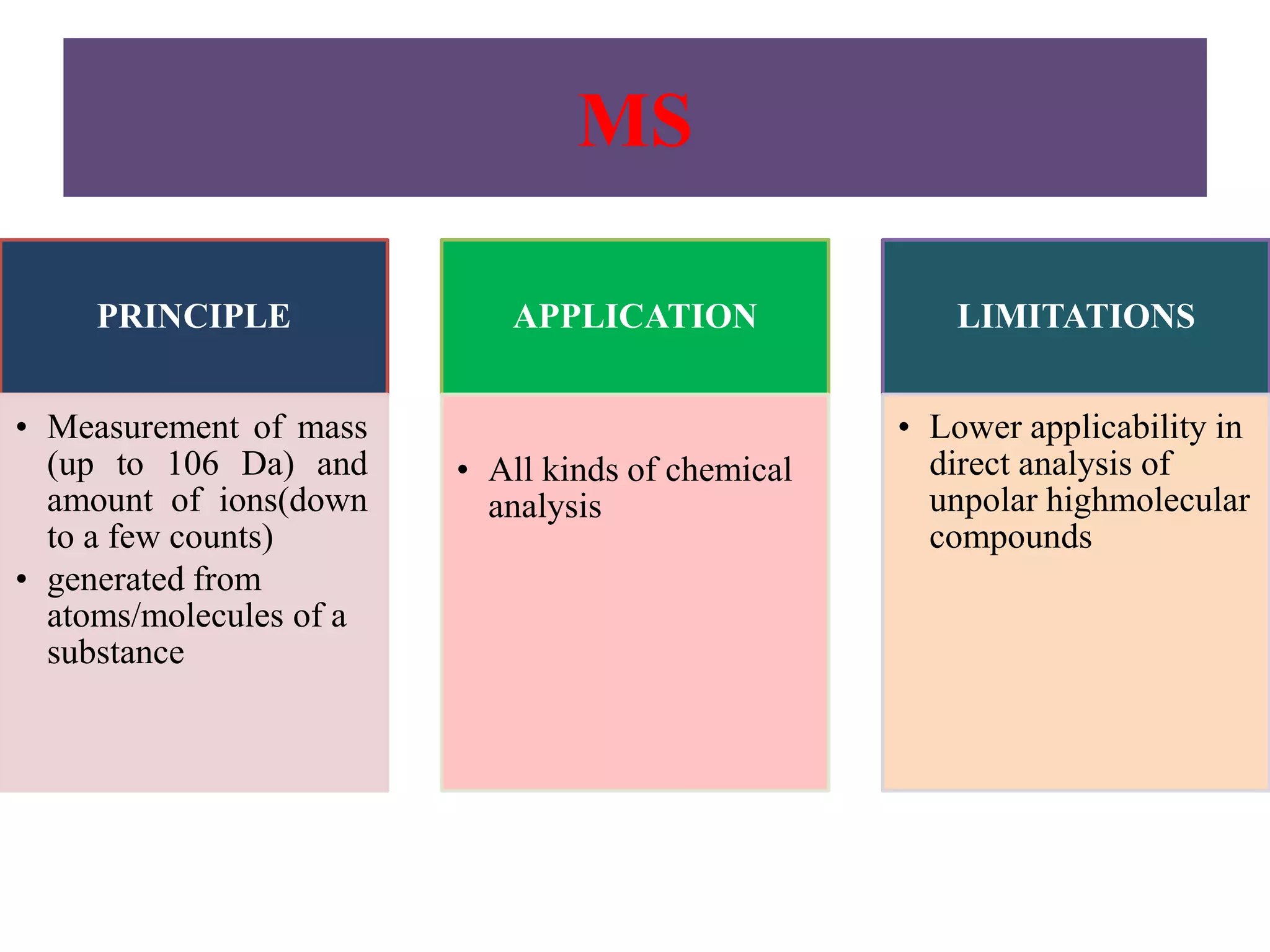
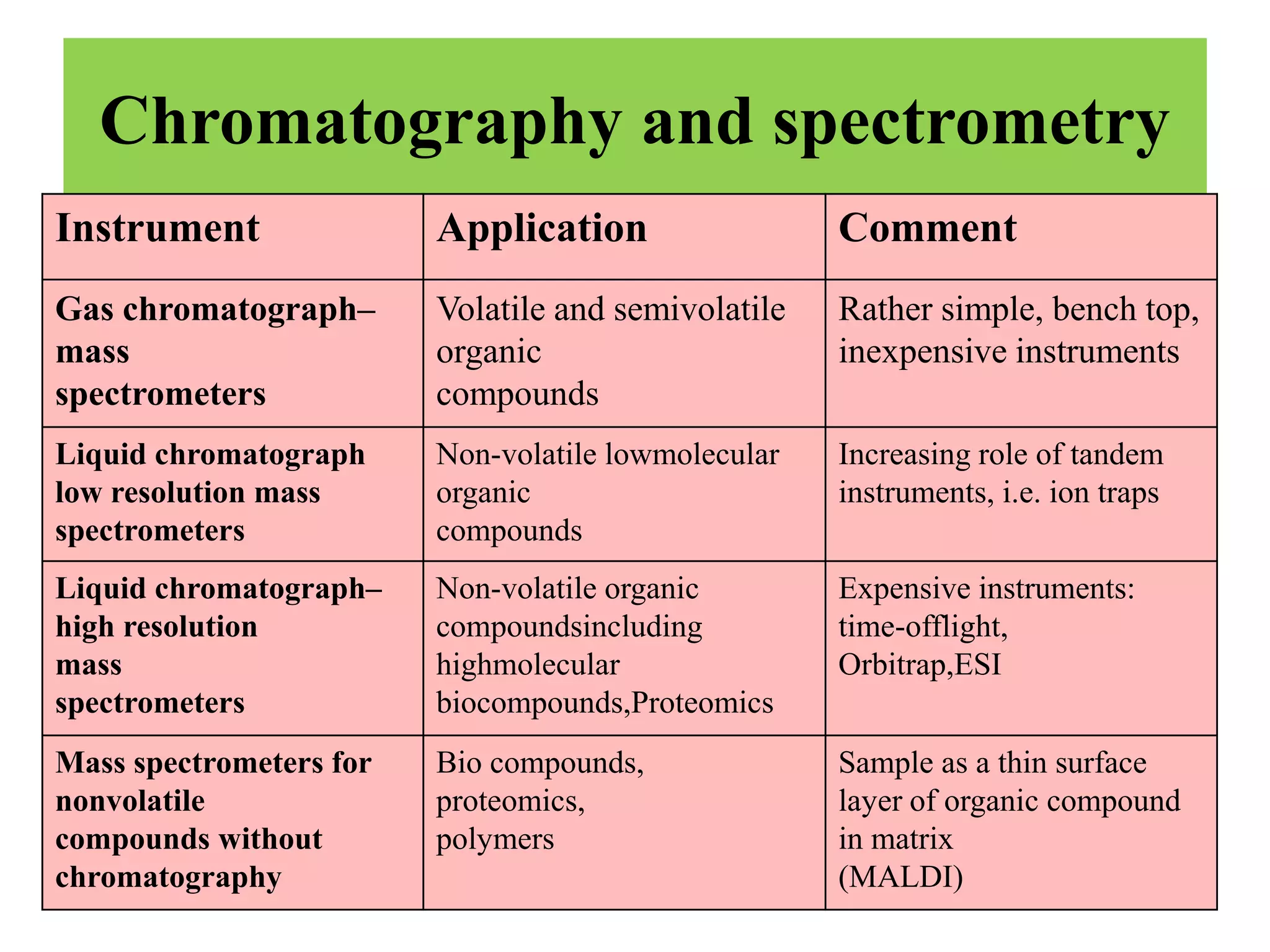
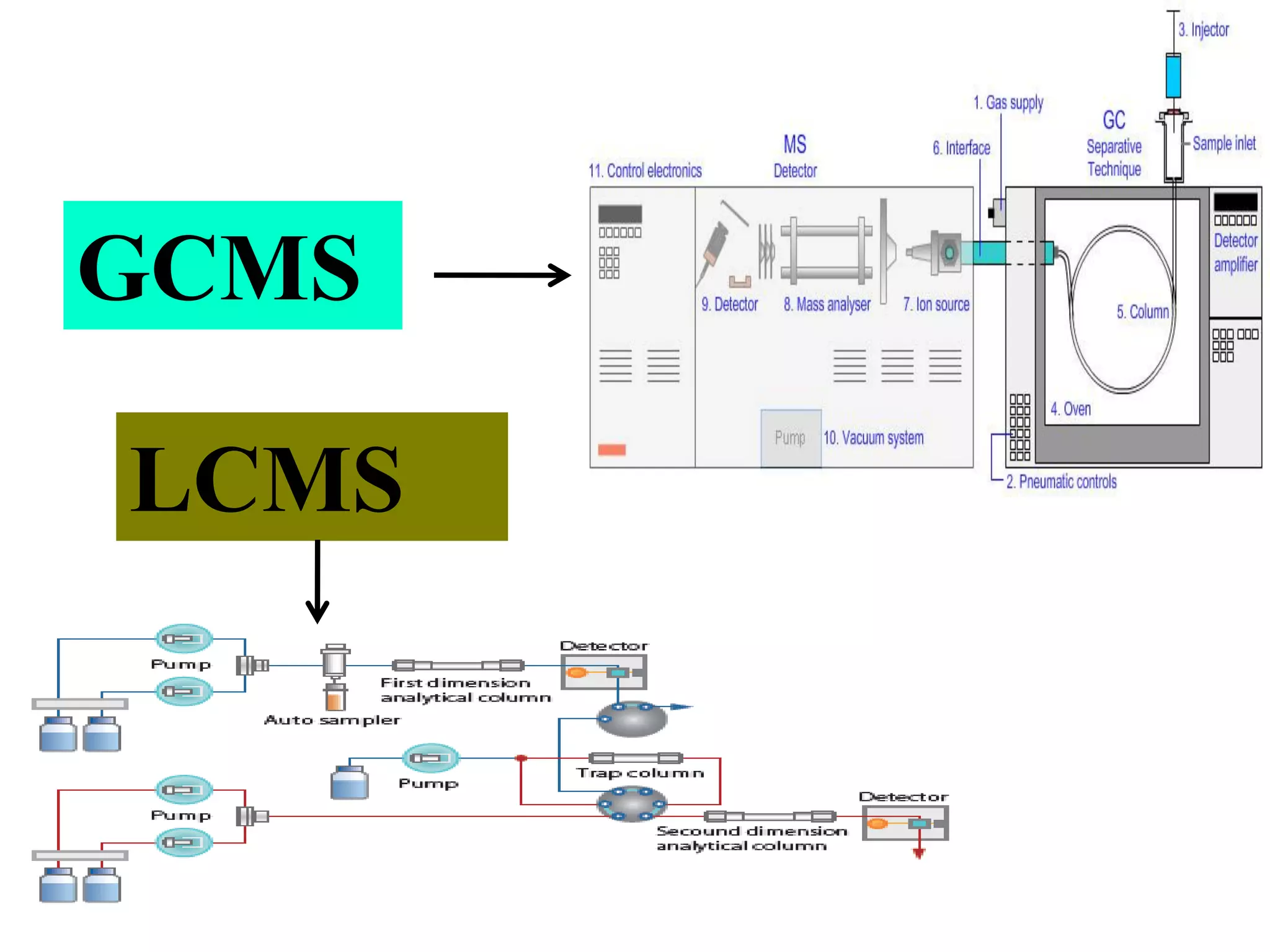
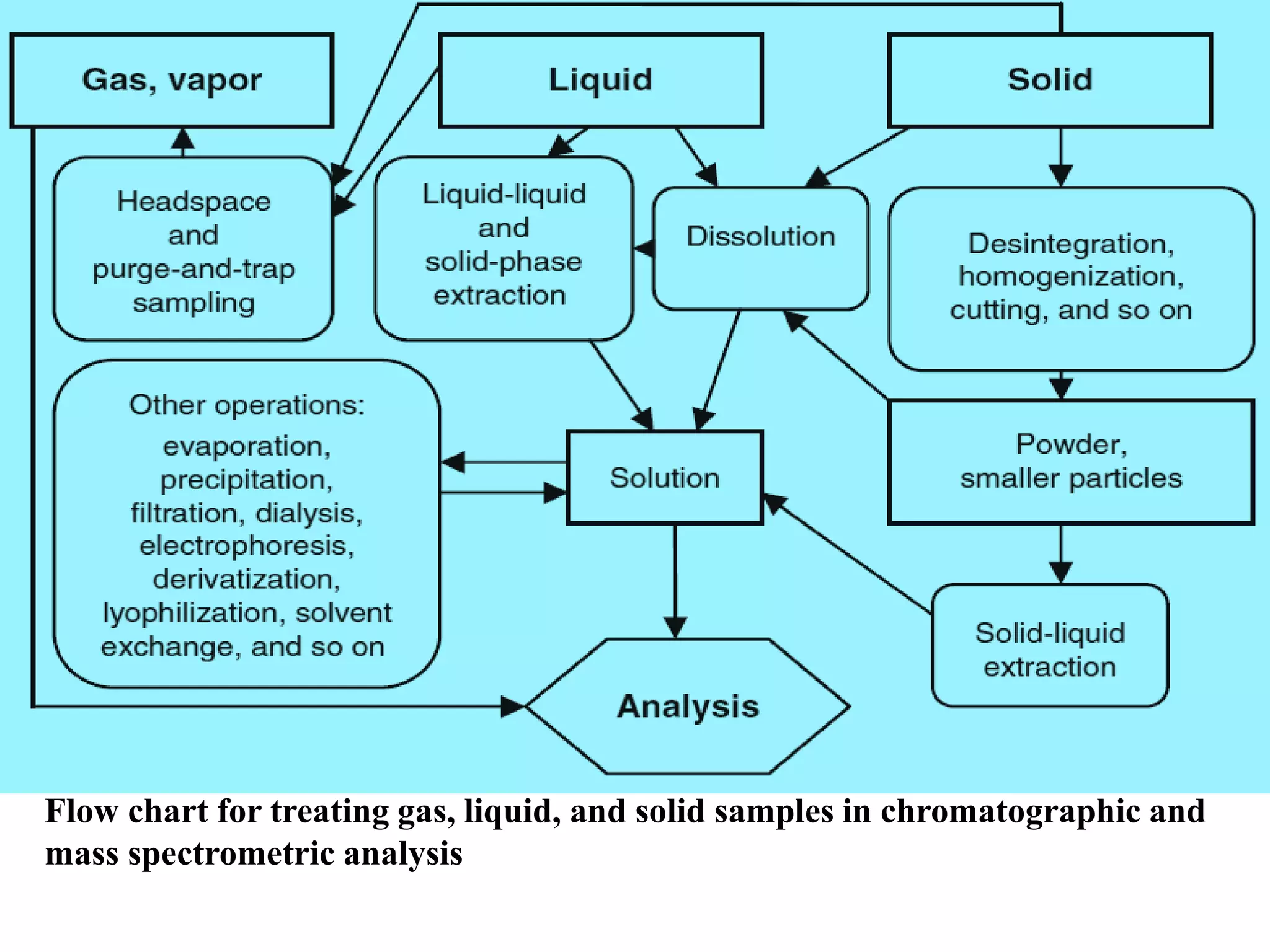
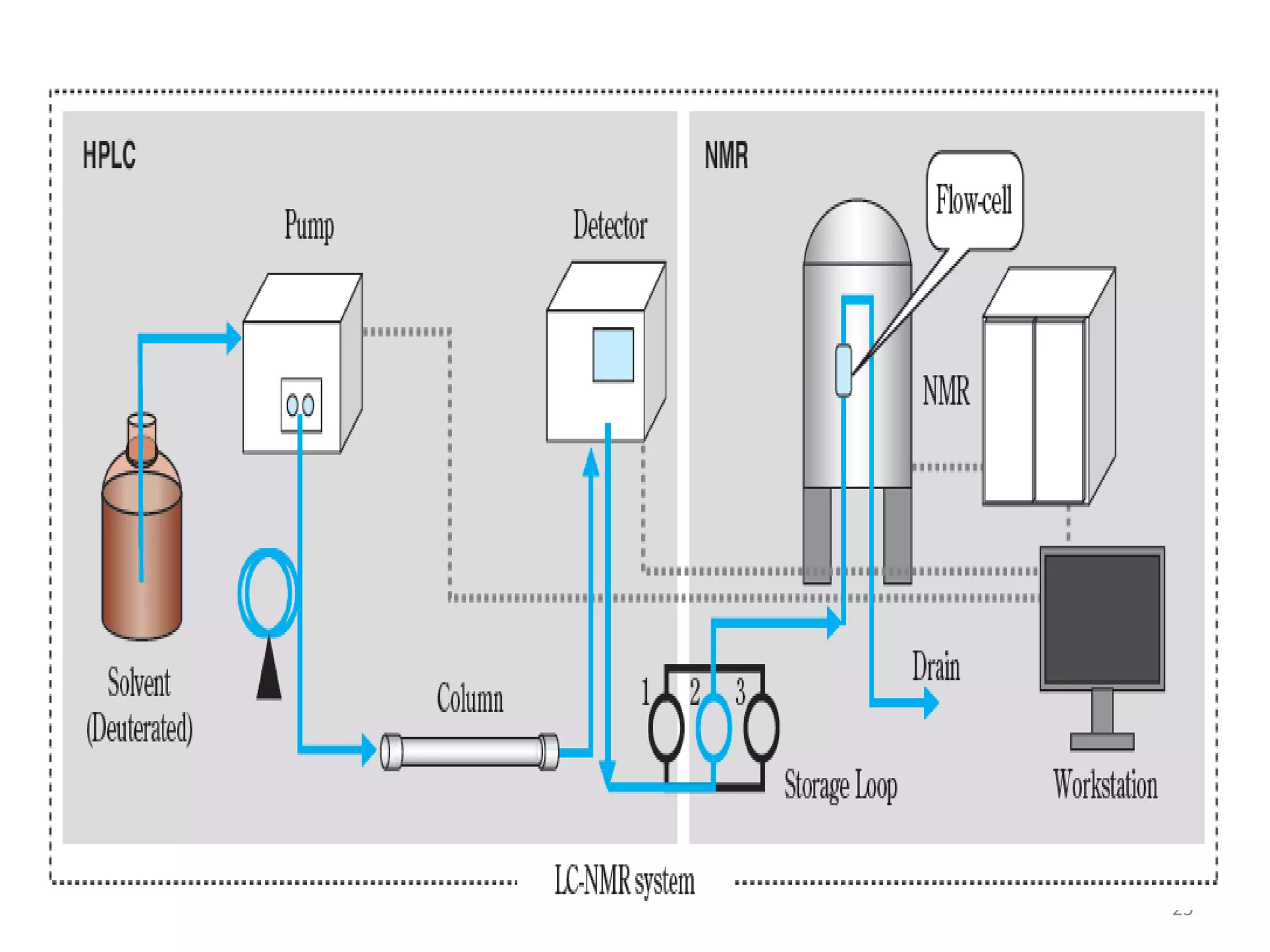
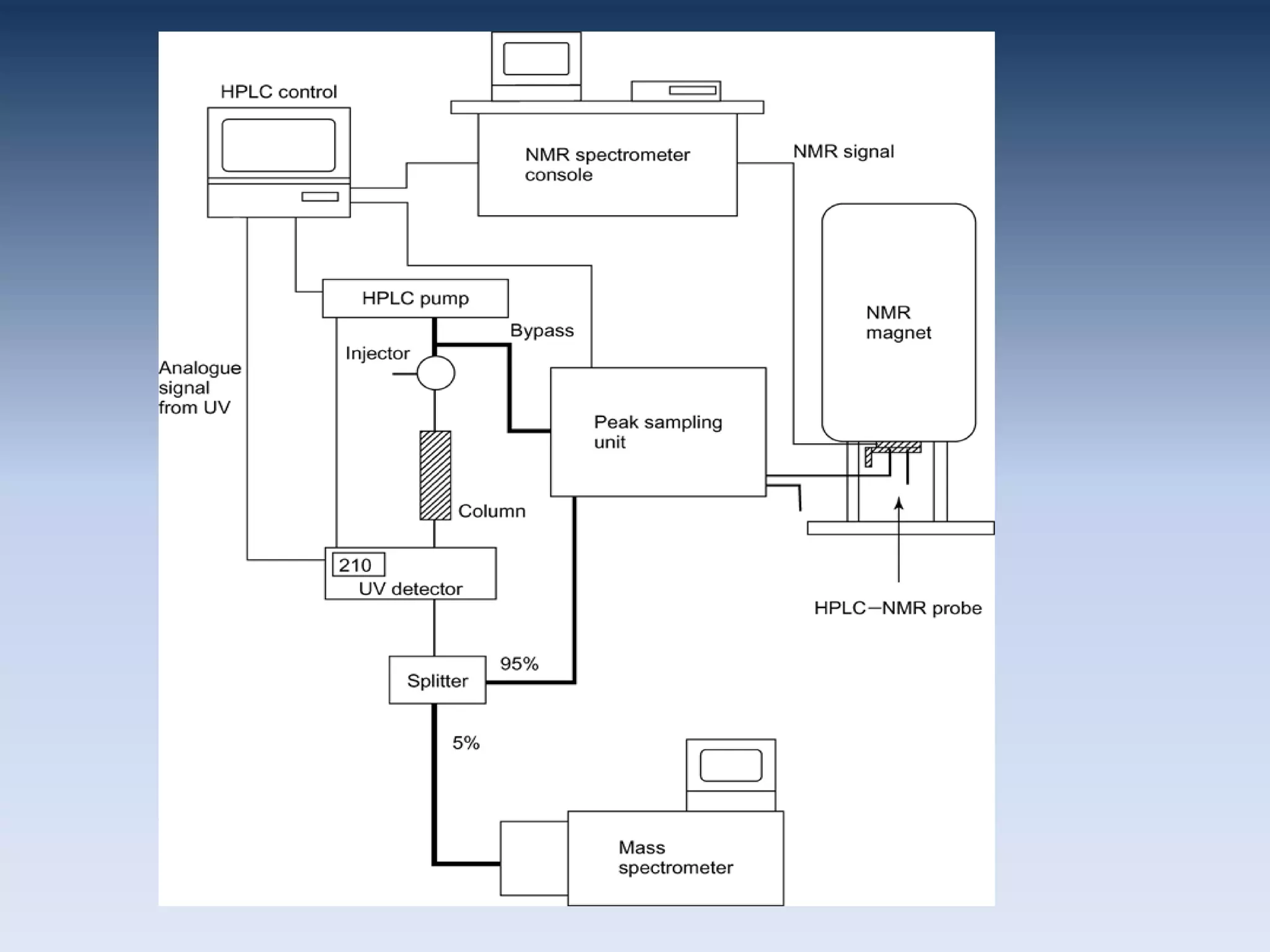
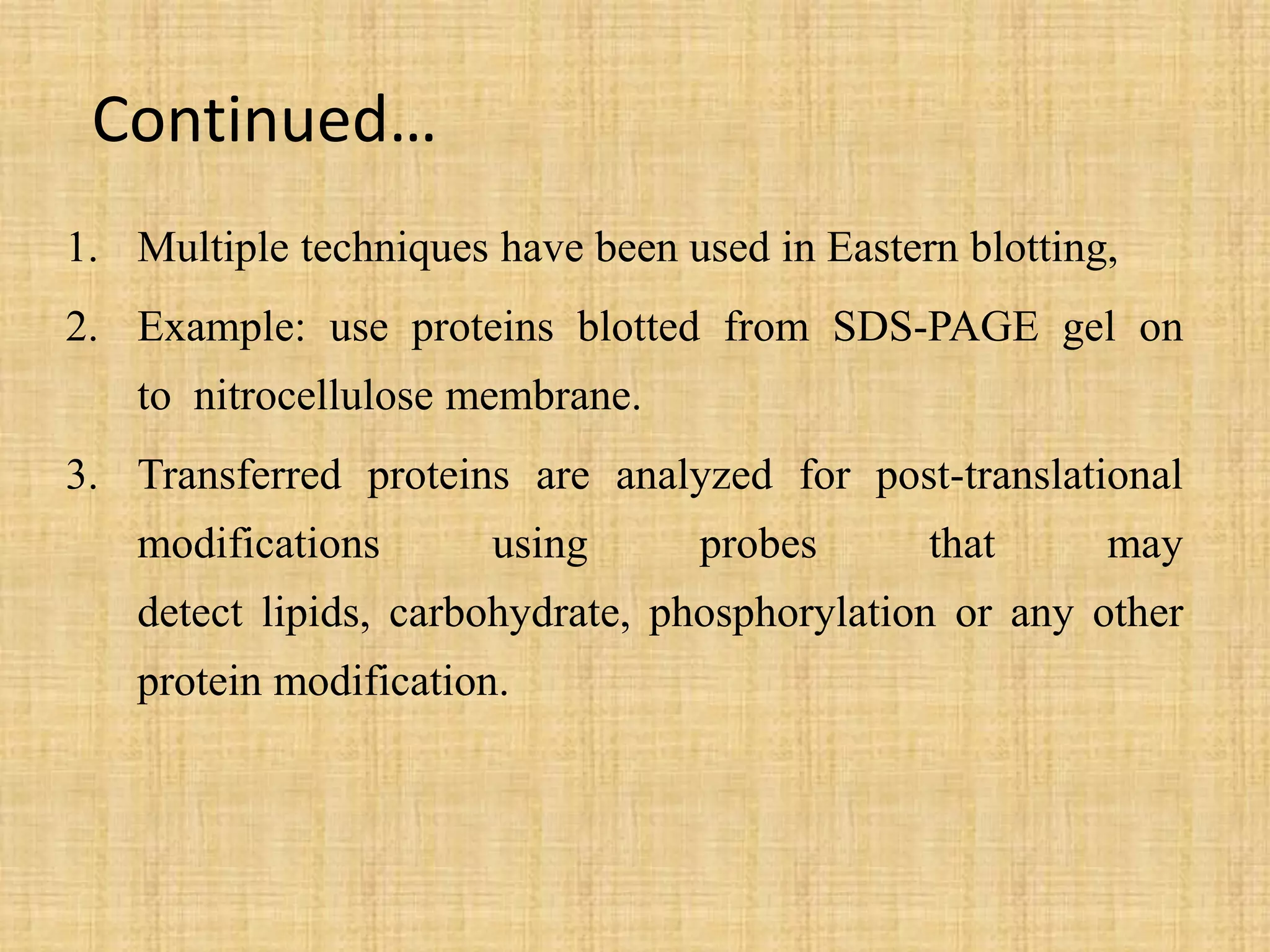
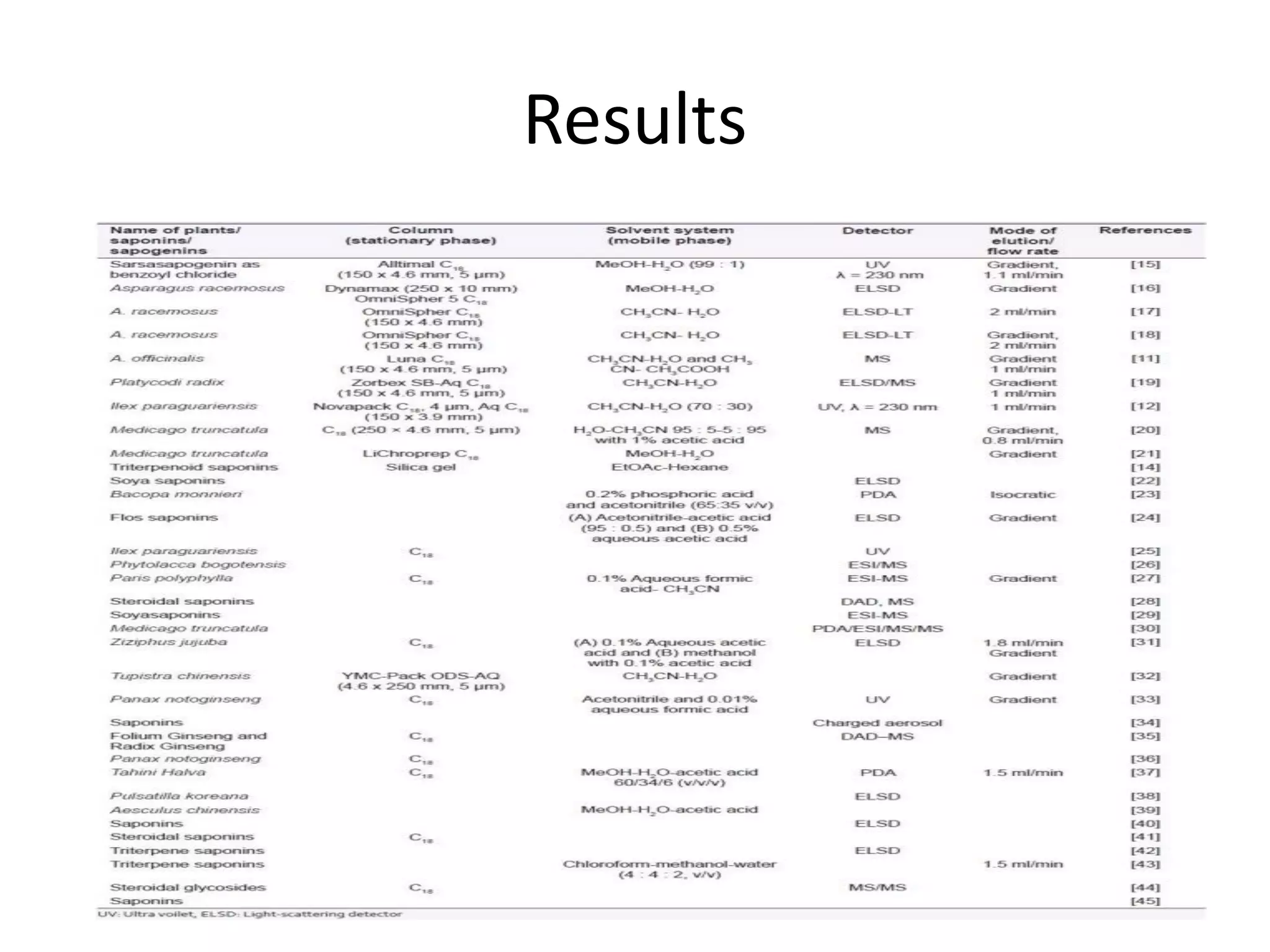
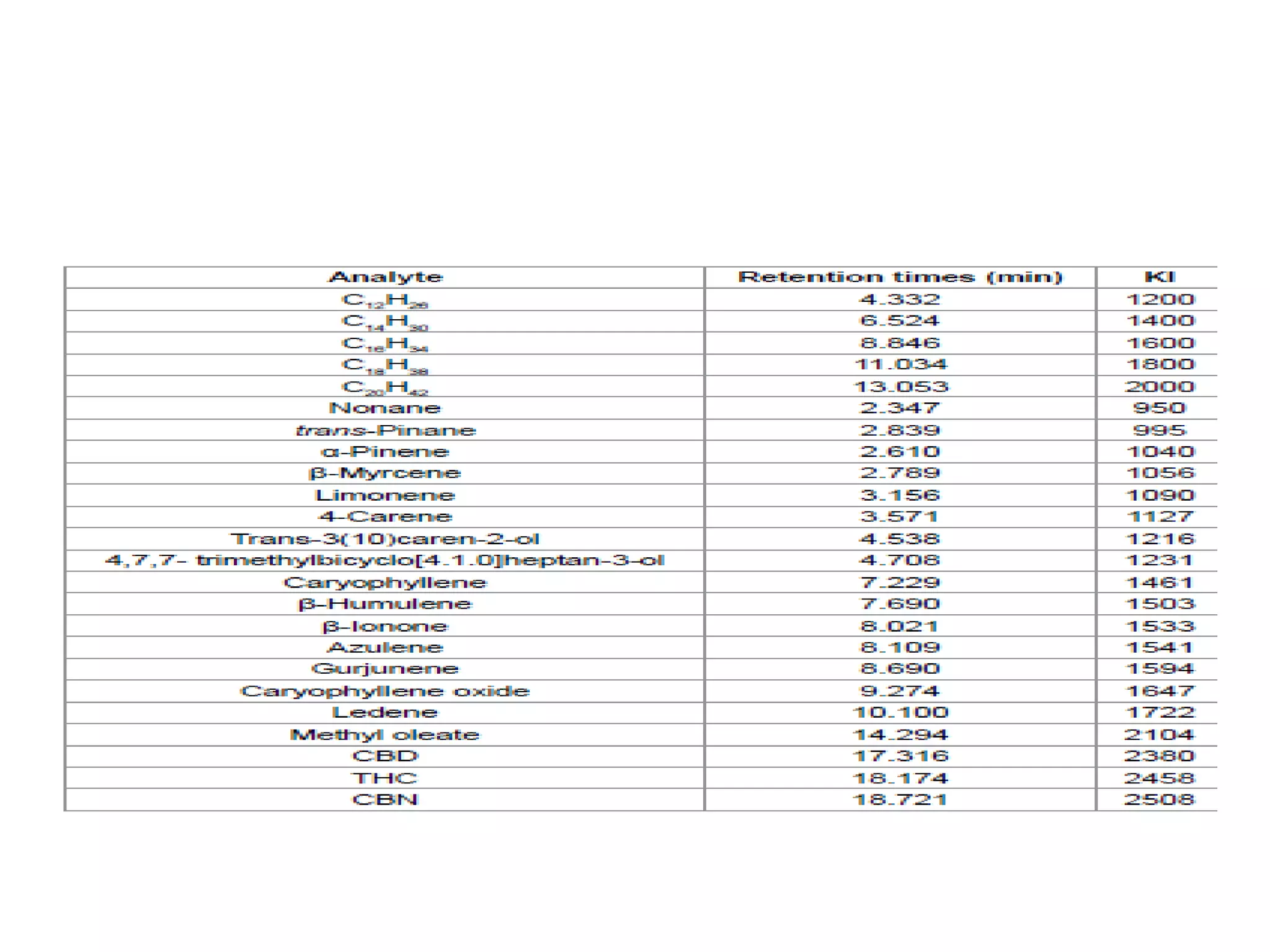
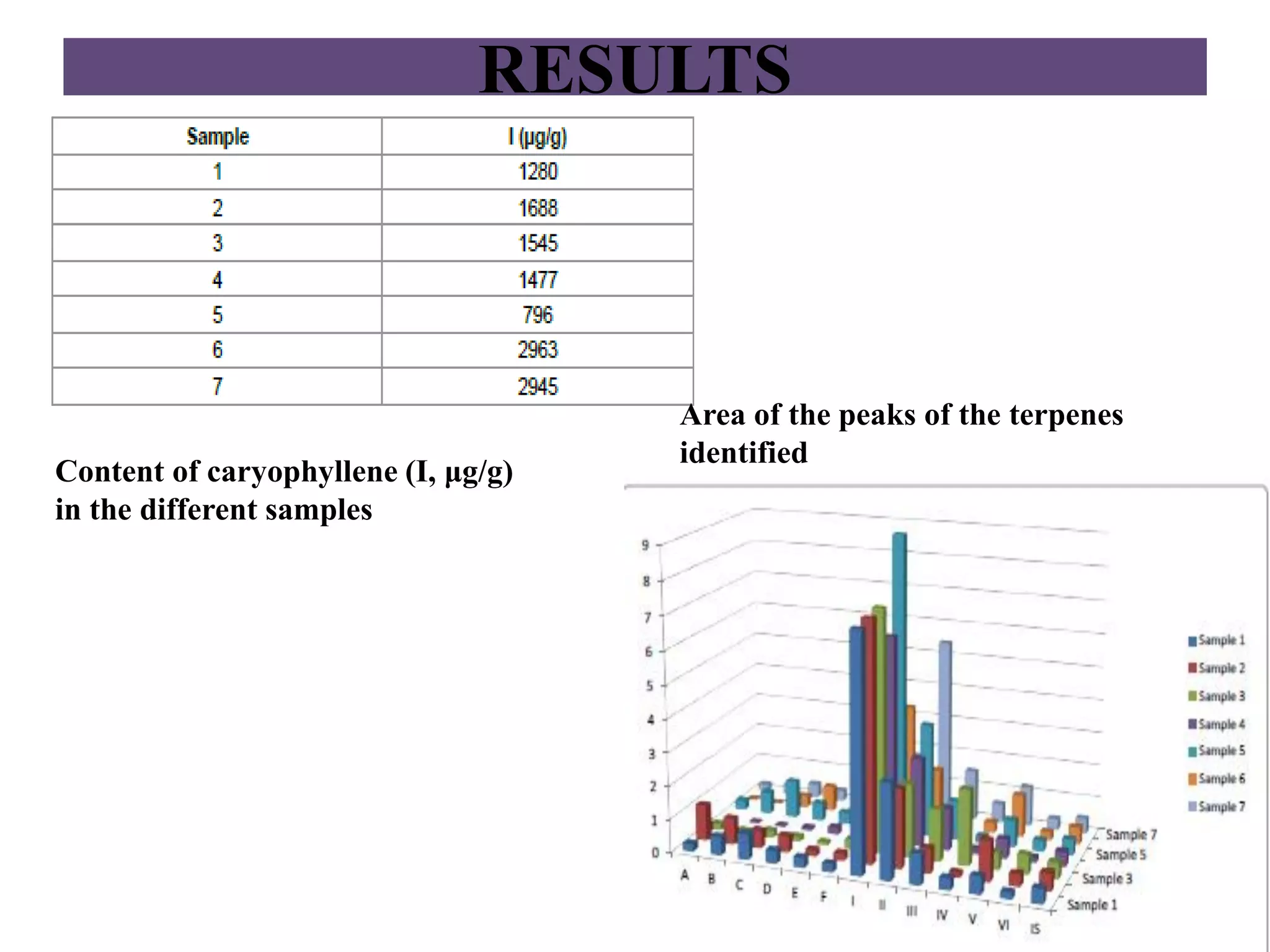
Upasana Mohapatra submitted a research paper on advanced techniques for analysis of organic compounds. The paper described several techniques including chromatography-spectrometry combinations like HPLC-NMR that allow identification of organic molecules. Elemental analysis, electrochemistry, chromatography, and molecular spectrometry each provide different information for organic compound analysis. HPLC-NMR in particular allows complex mixtures to be separated and identified using NMR. The techniques discussed provide powerful tools for identification of organic compounds in various applications like metabolite analysis.