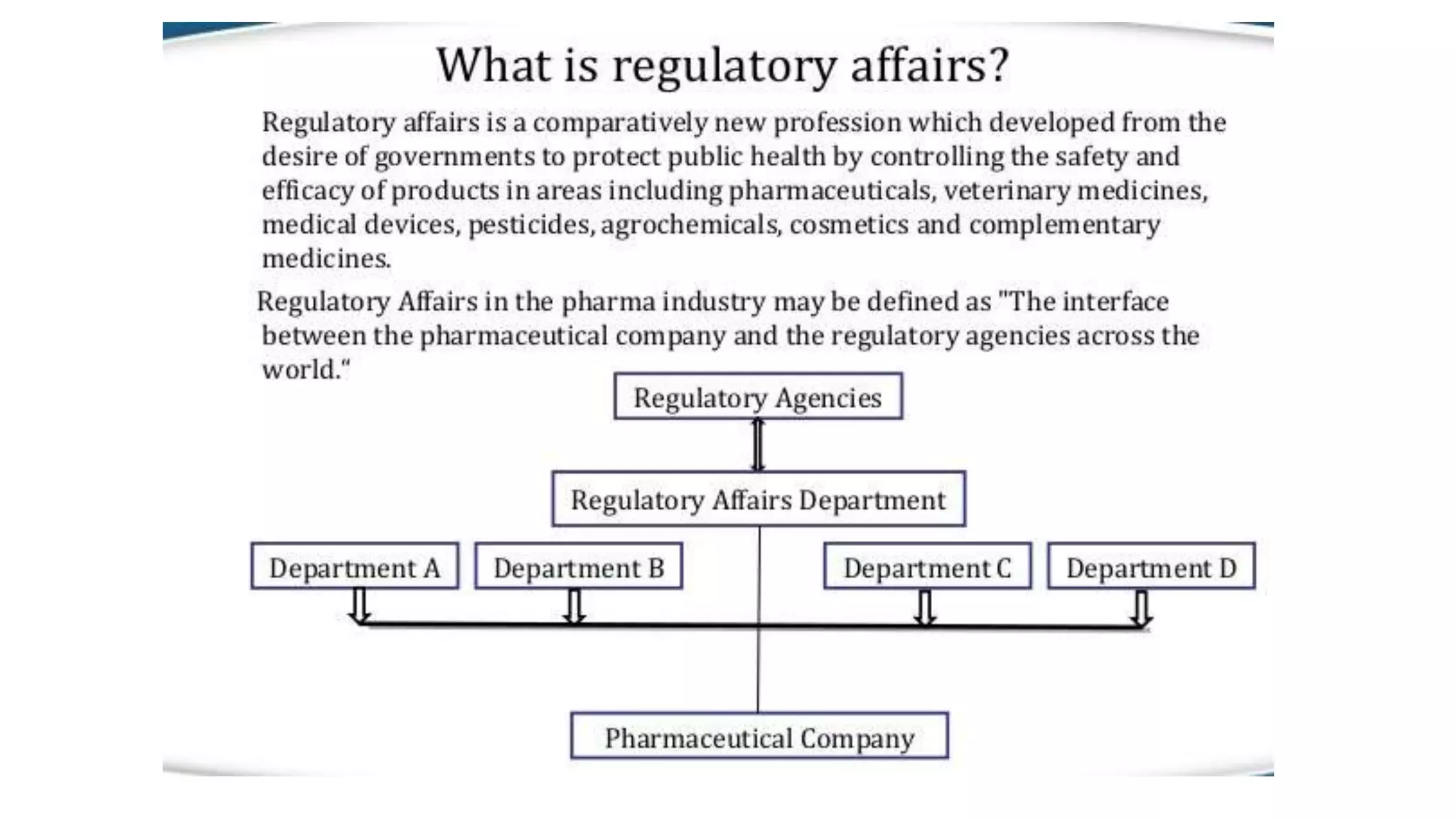
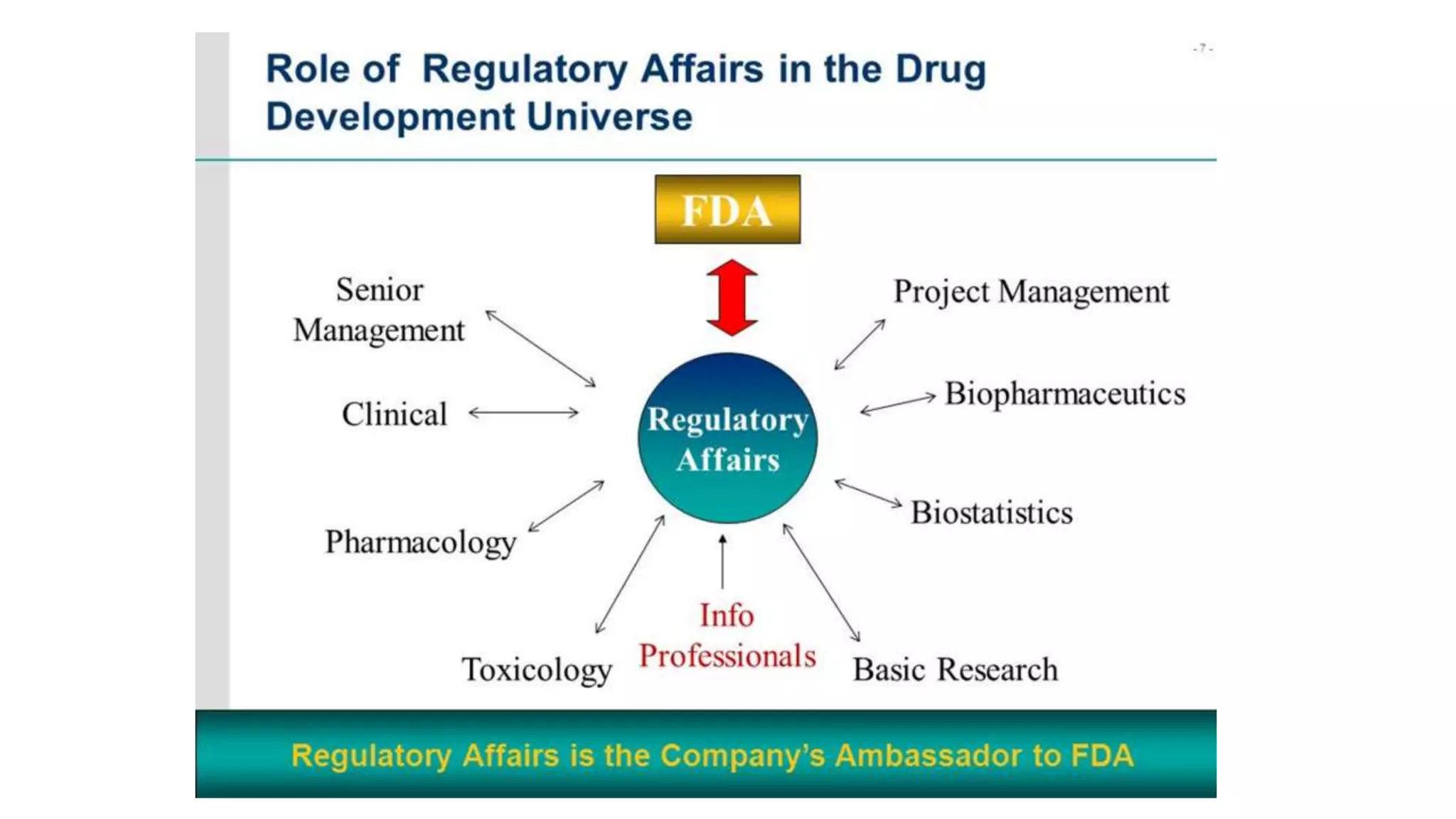
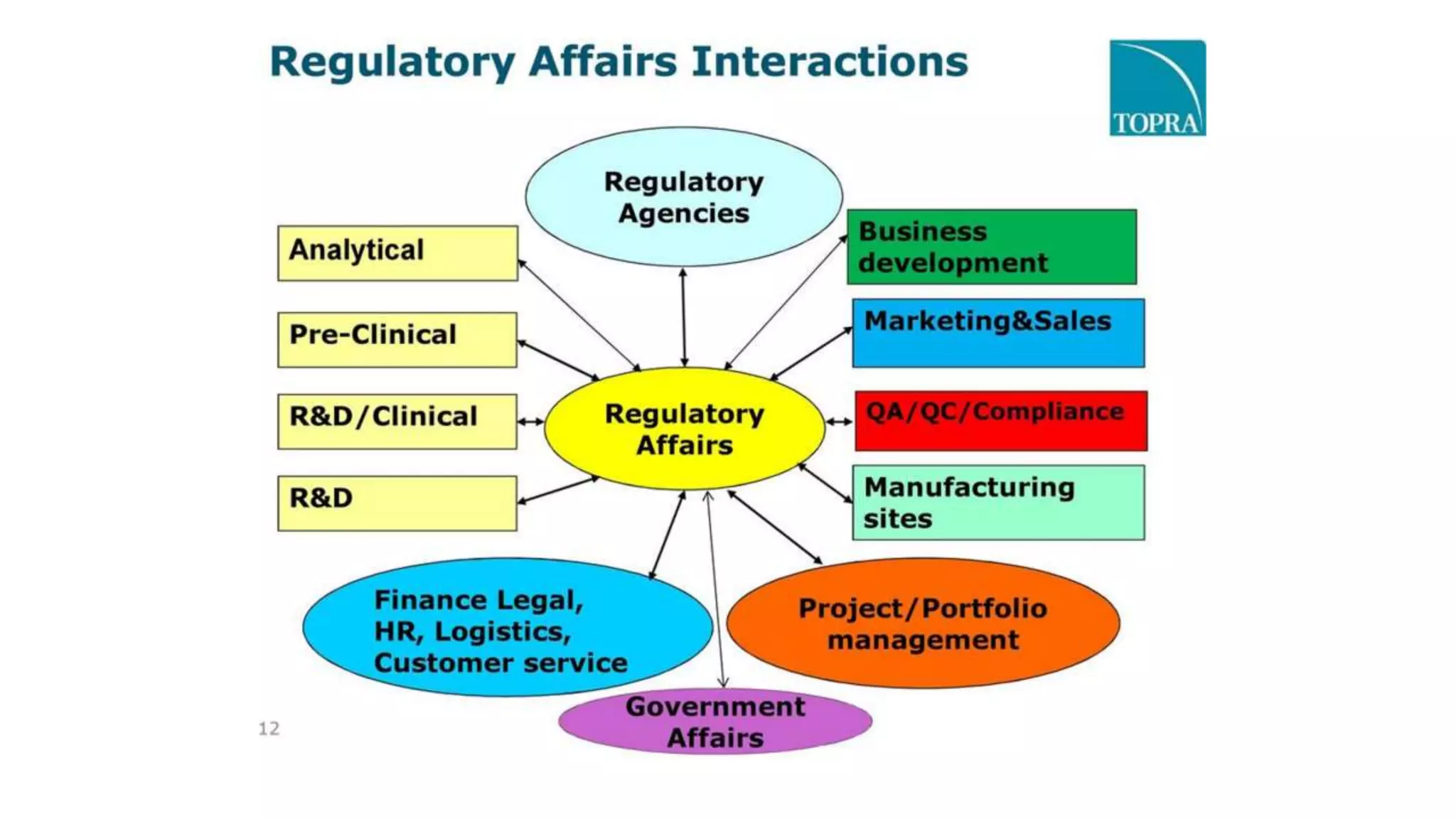
Regulatory affairs plays a crucial role in the pharmaceutical industry and is involved in all stages of drug development as well as after drug approval and marketing. Regulatory affairs professionals ensure that products contribute to public health by controlling safety and efficacy through compliance with regulations. They provide strategic and technical advice throughout development and registration of products with regulatory agencies. The scope of regulatory affairs includes pharmaceuticals, medical devices, diagnostics, biologics, and other health-related products.