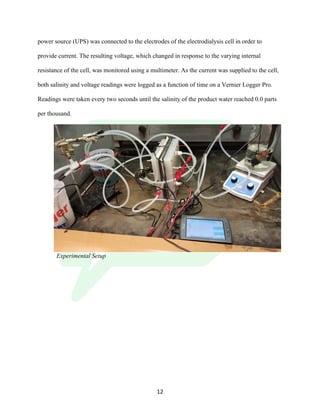
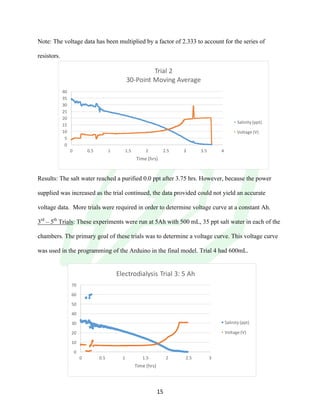
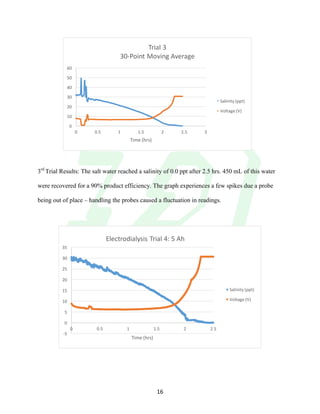
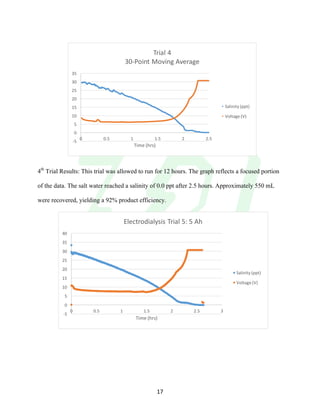
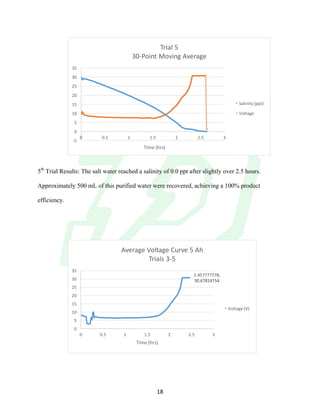
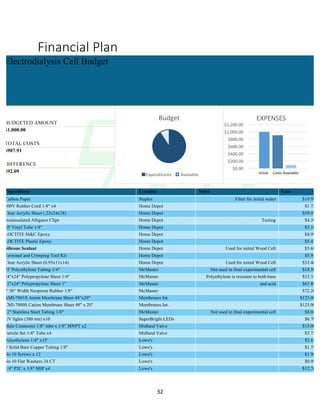
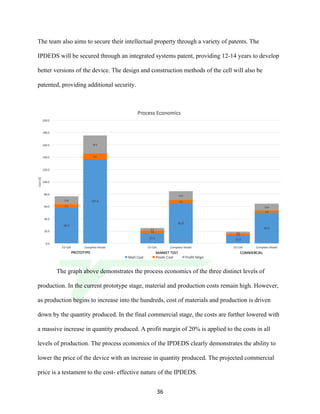
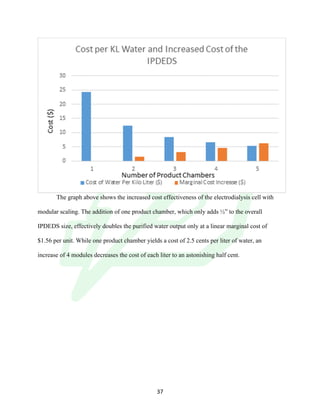
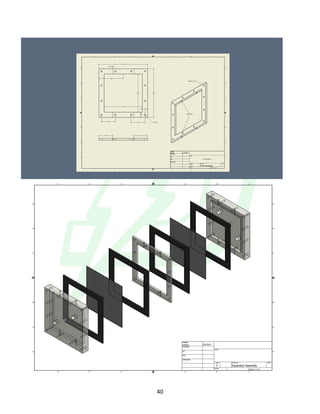
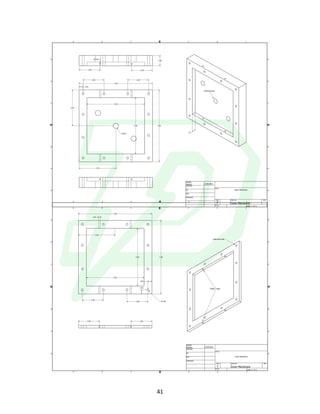
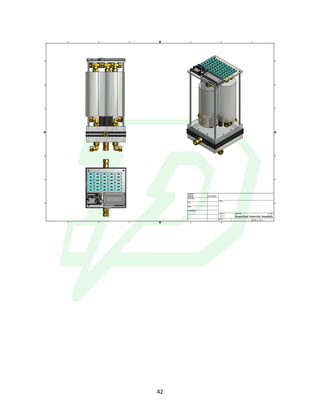
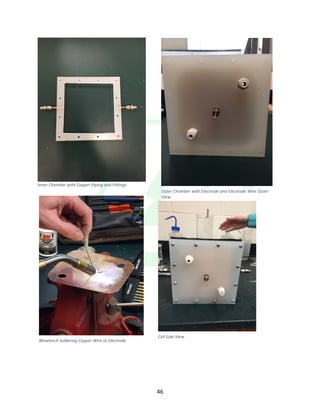
The document describes the development of an integrated power delivery electrodialysis desalination system (IPDEDS) by a team of students. The IPDEDS uses electrodialysis, a membrane process, to remove salt from brackish and ocean water in an energy efficient manner. It is powered independently by a photovoltaic cell, requiring minimal energy. The team designed and tested plastic electrodialysis cells that successfully removed salt from saltwater samples. Their goal is for the IPDEDS to benefit water-stressed coastal communities and emergency situations by providing portable, low-cost desalination.