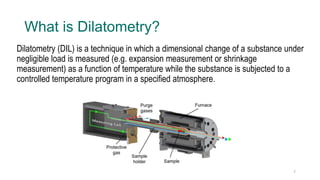
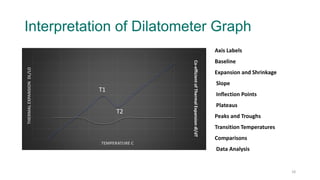
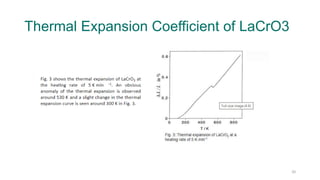
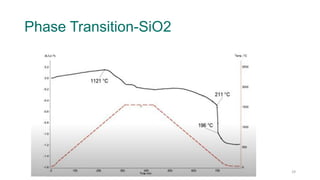
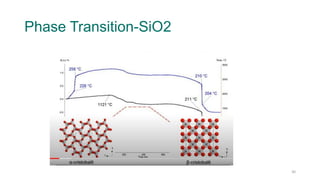
The document provides an overview of dilatometry, a technique used to measure dimensional changes in materials due to thermal expansion under controlled temperature conditions. It discusses the experimental setup, types of samples (metals, ceramics, polymers, etc.), measurement techniques, and applications including the determination of thermal expansion coefficients and phase transitions. Additionally, it covers data interpretation, including graphical analysis of thermal expansion and density changes.