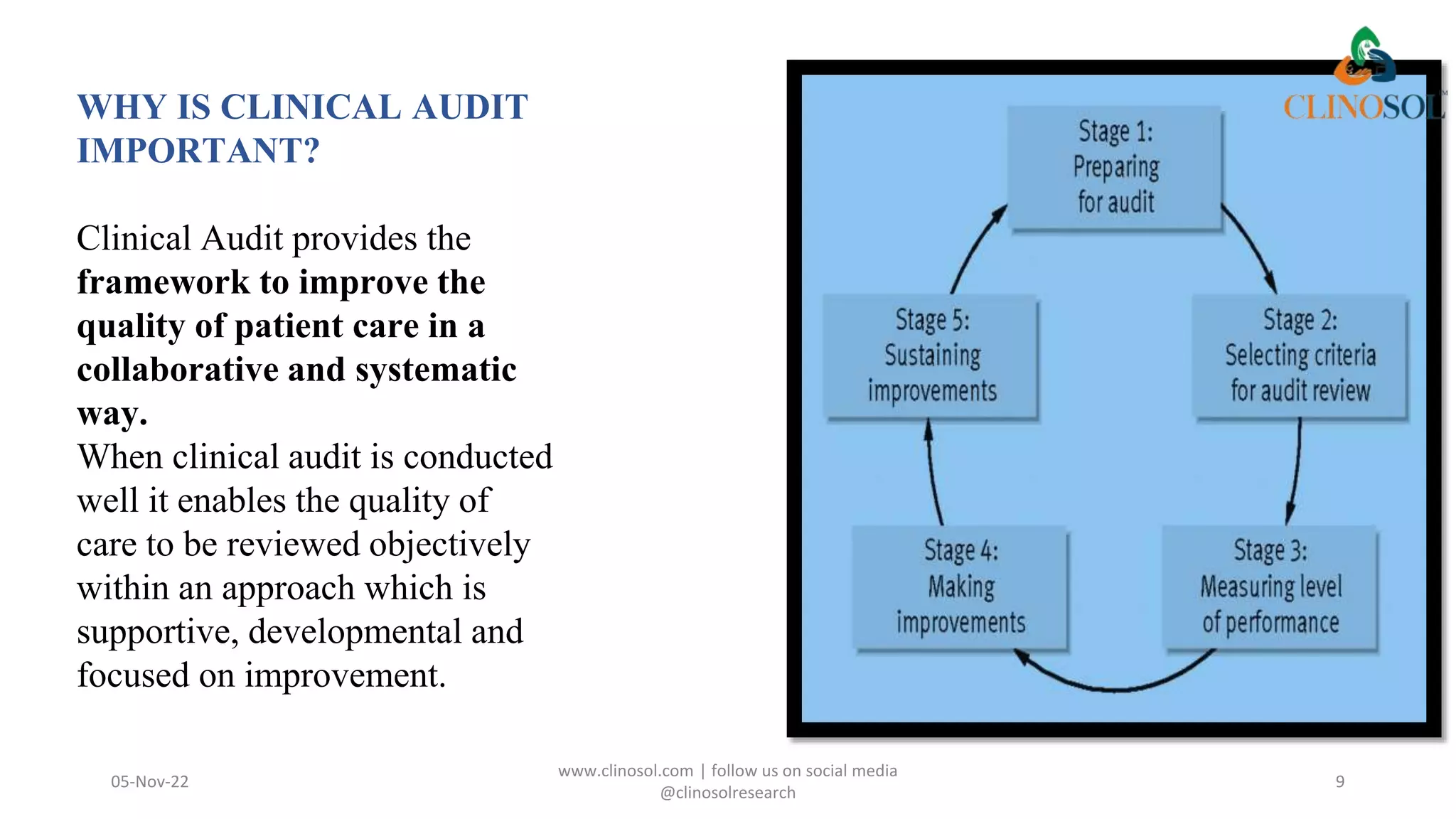
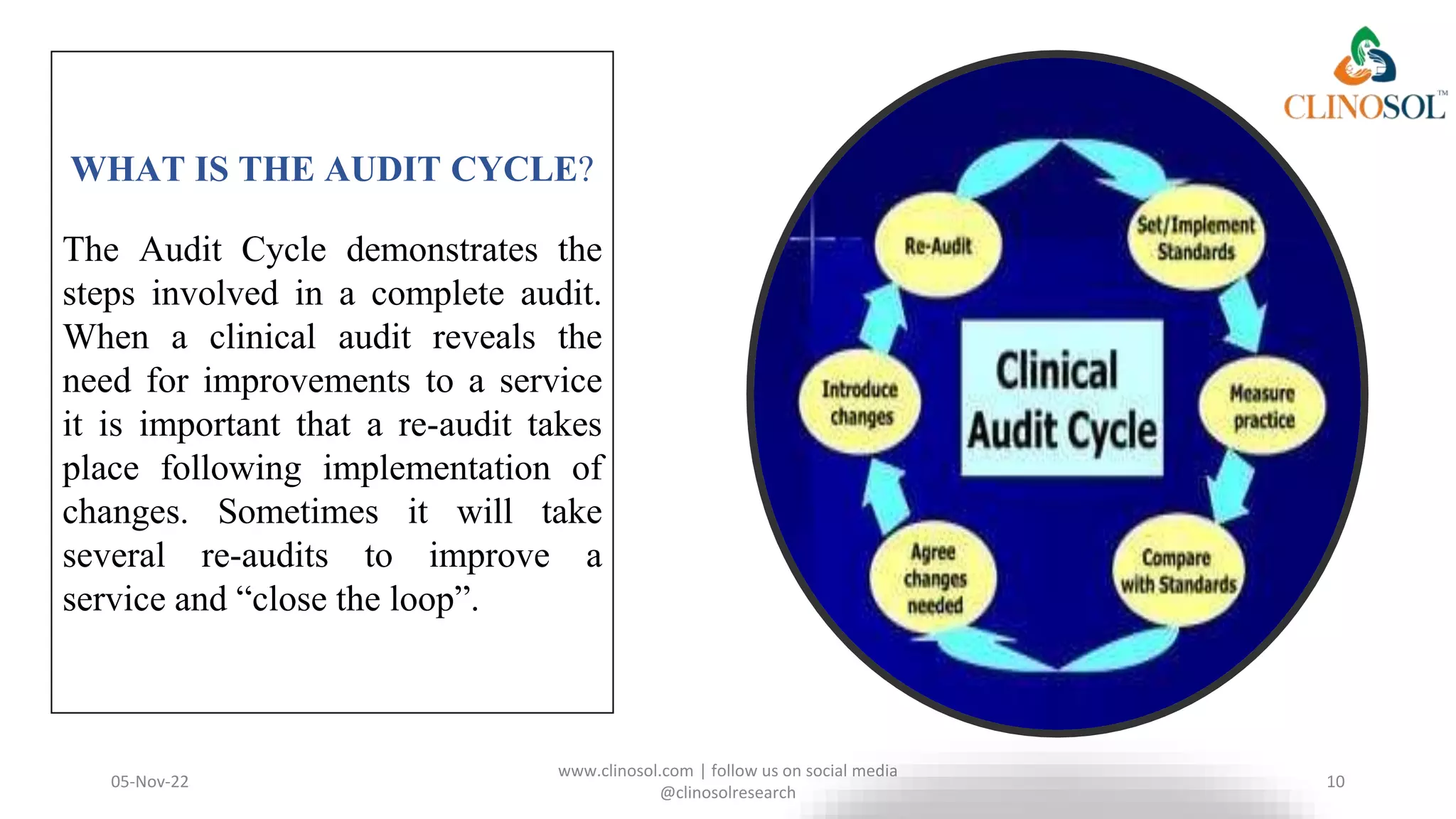
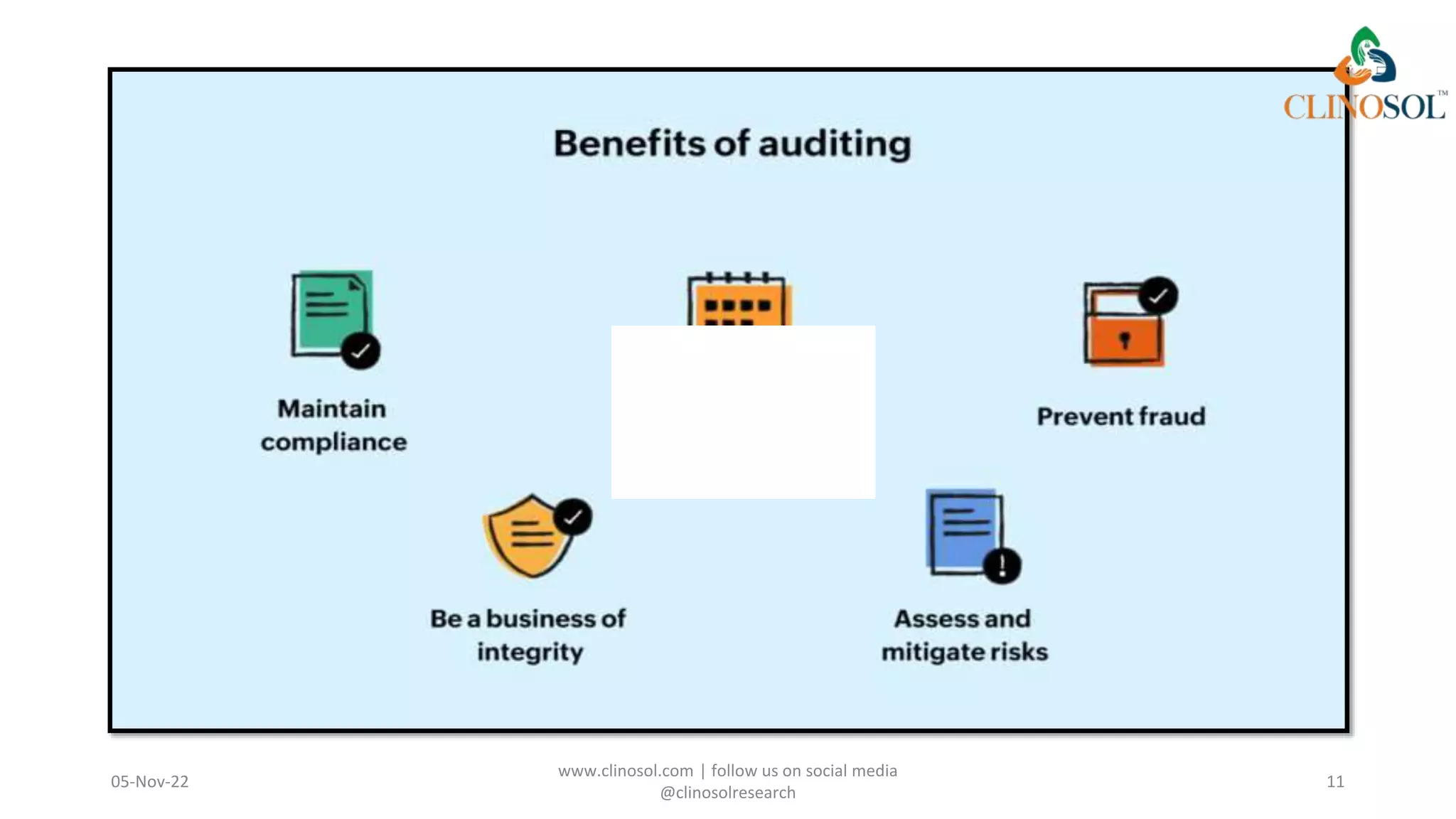
The document discusses the key differences between monitoring and auditing in clinical trials, highlighting that monitoring is an ongoing management-directed process while auditing is a systematic evaluation performed by independent professionals. It emphasizes the importance of both processes in ensuring quality and compliance with regulatory standards, as well as the necessity of documenting qualifications for those involved. Clinical audits are framed as essential for quality improvement in patient care, involving collaboration and the engagement of patients in the process.