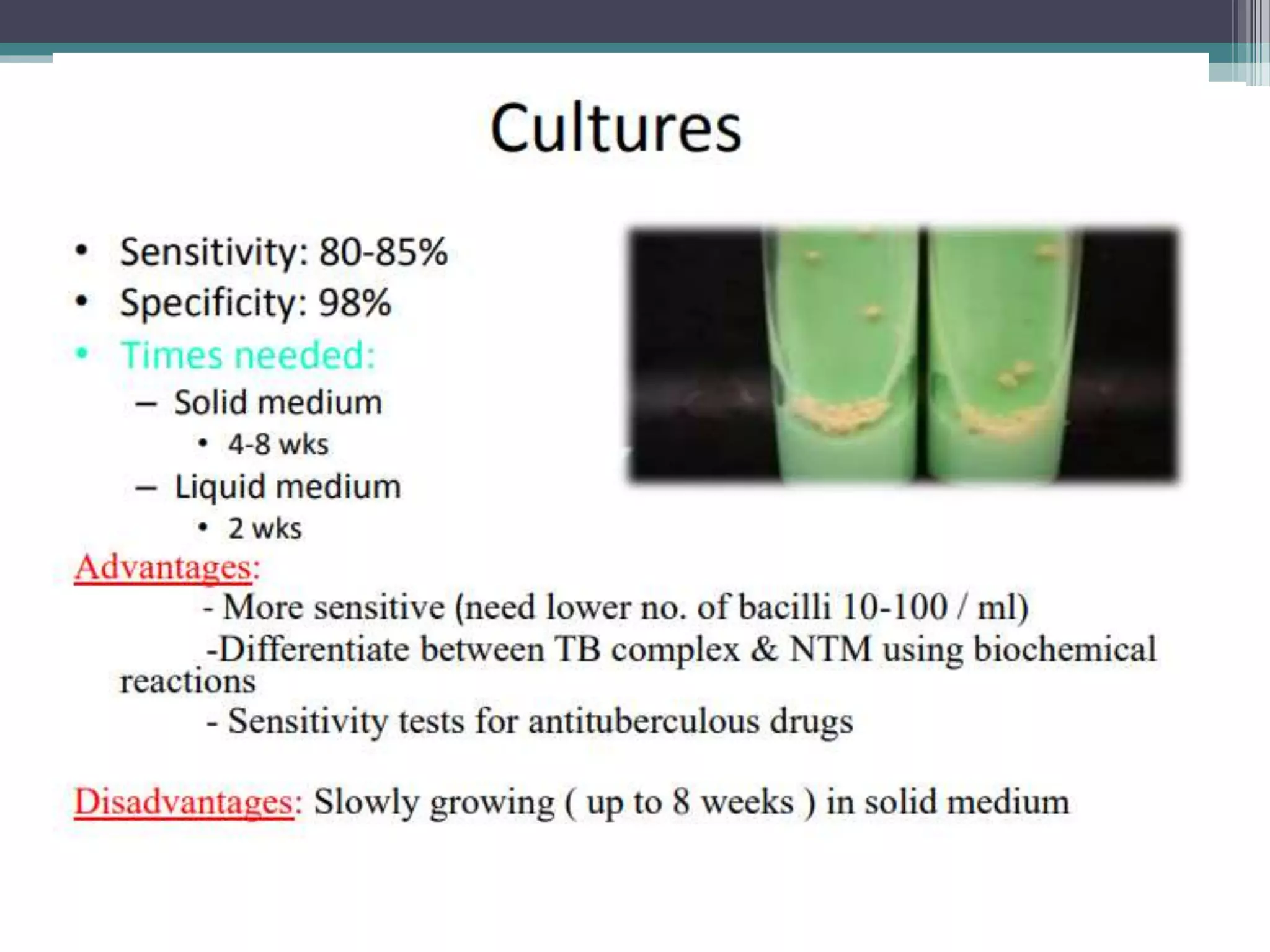
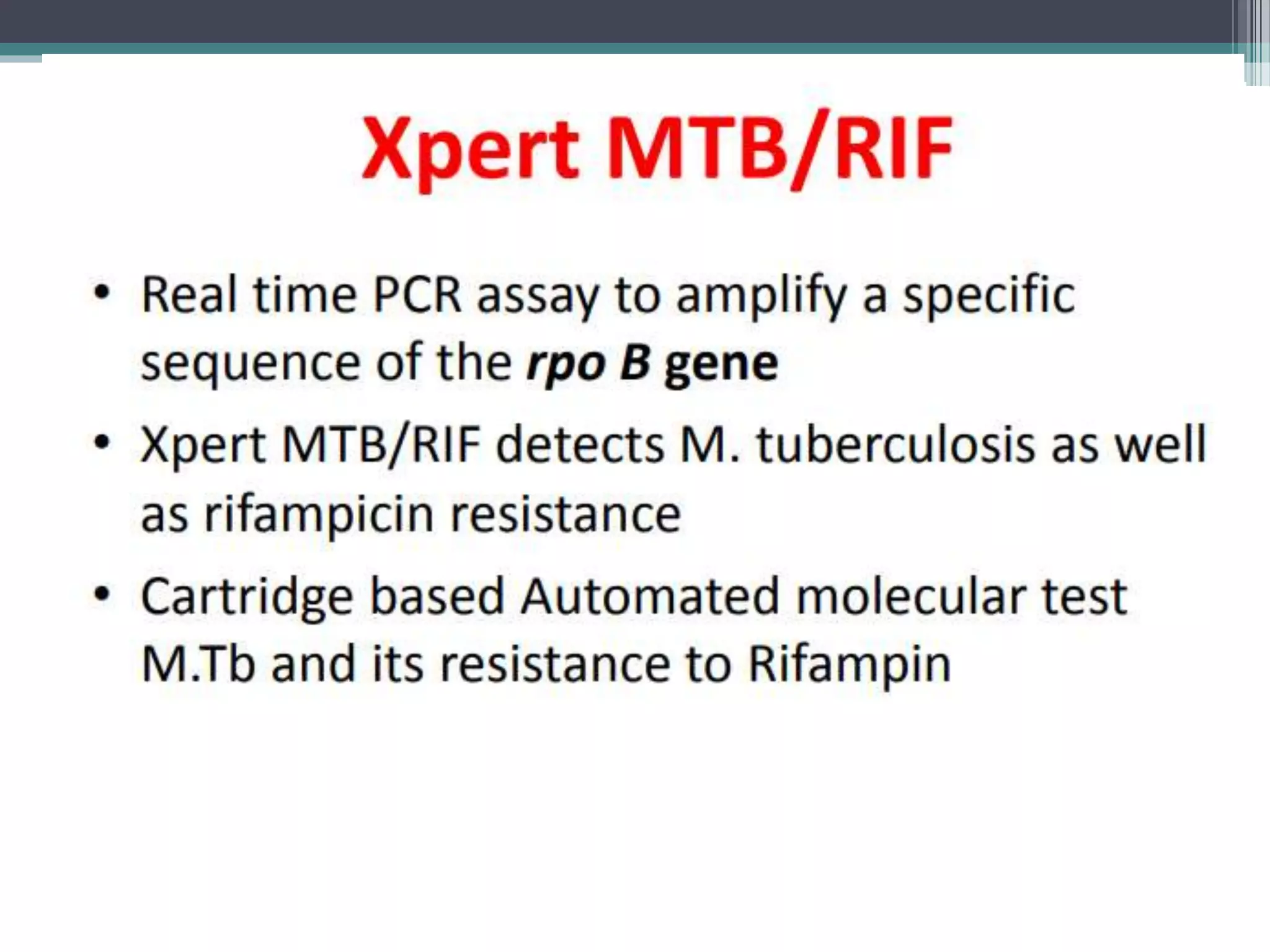
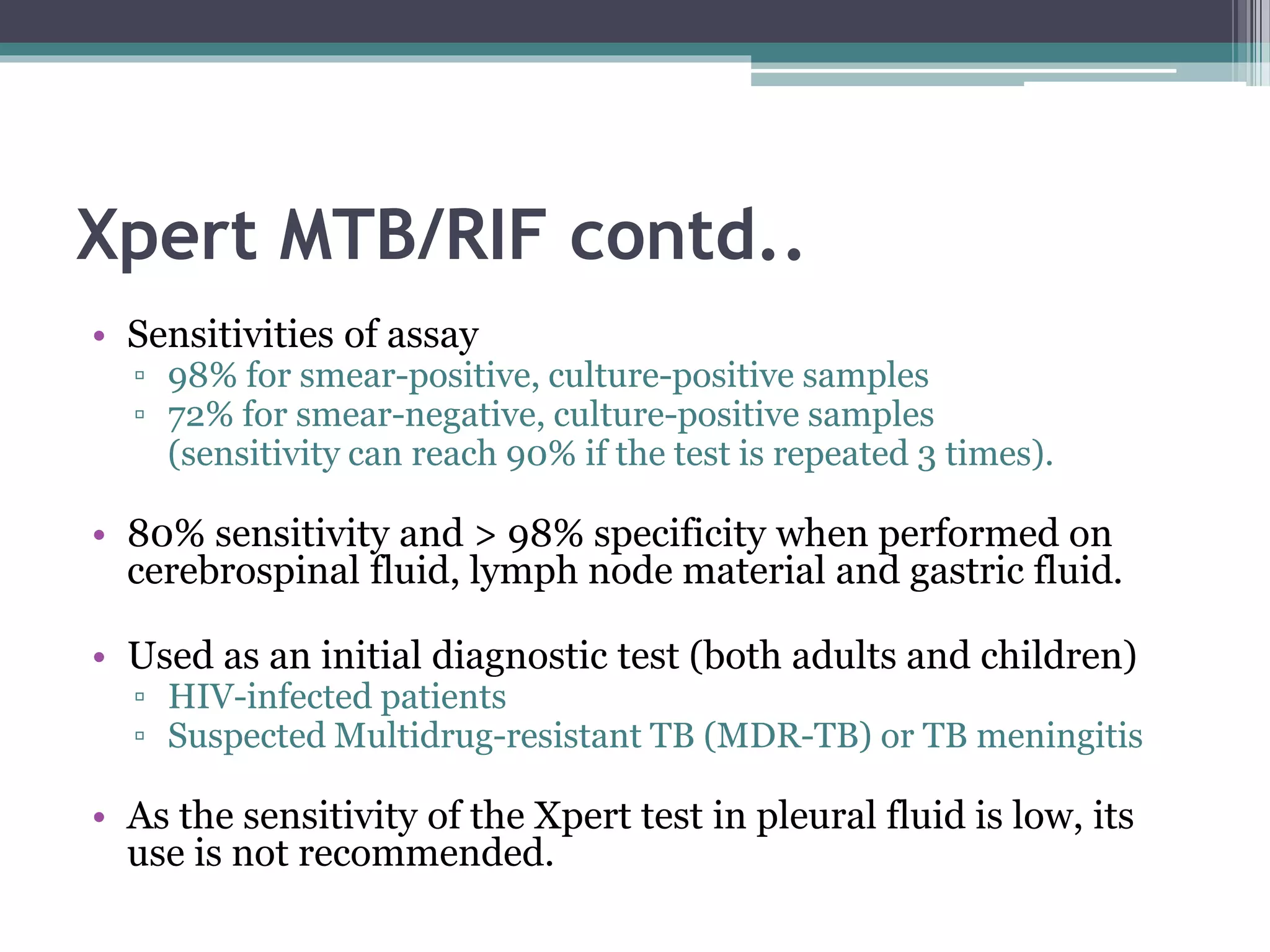
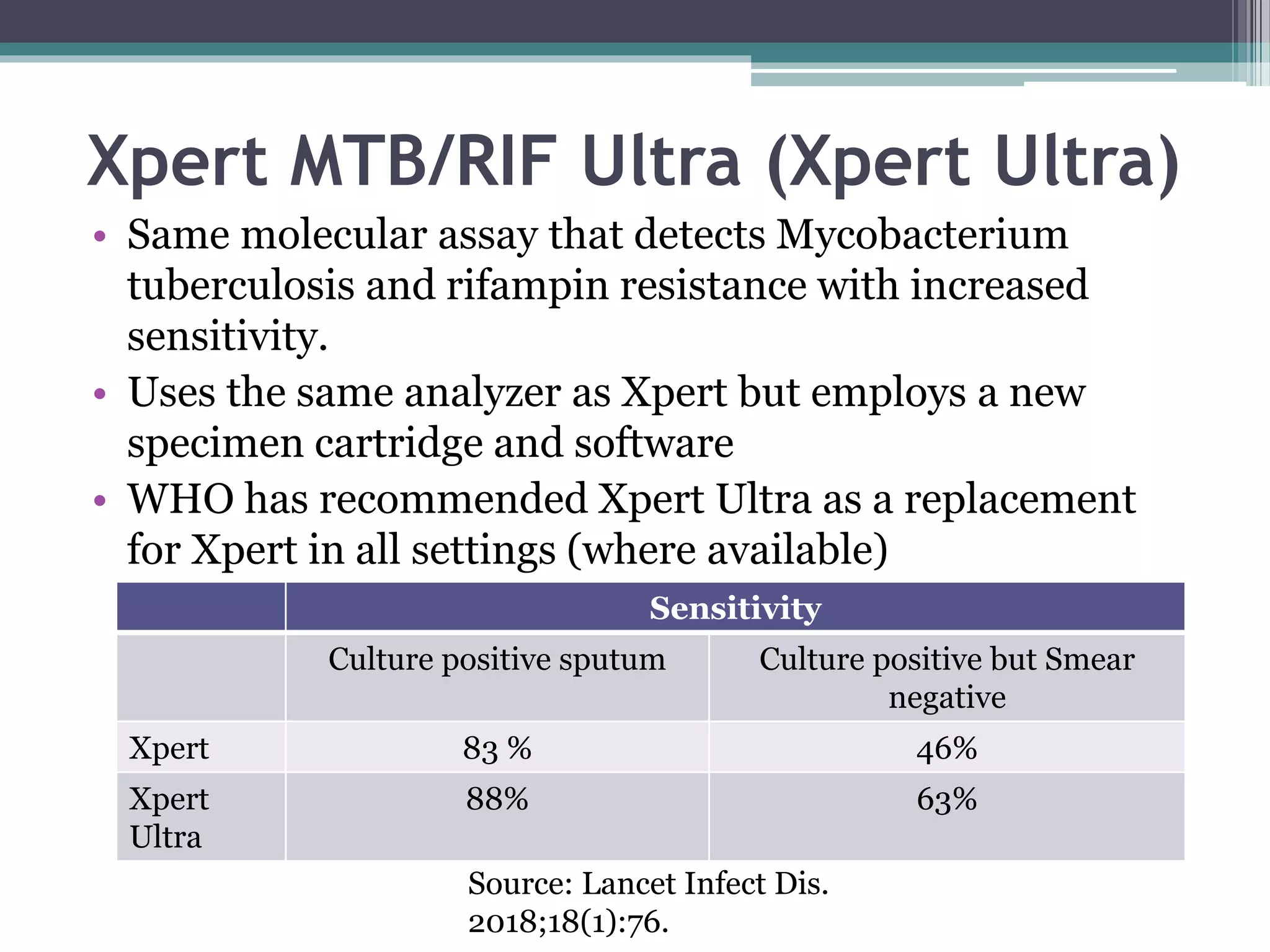
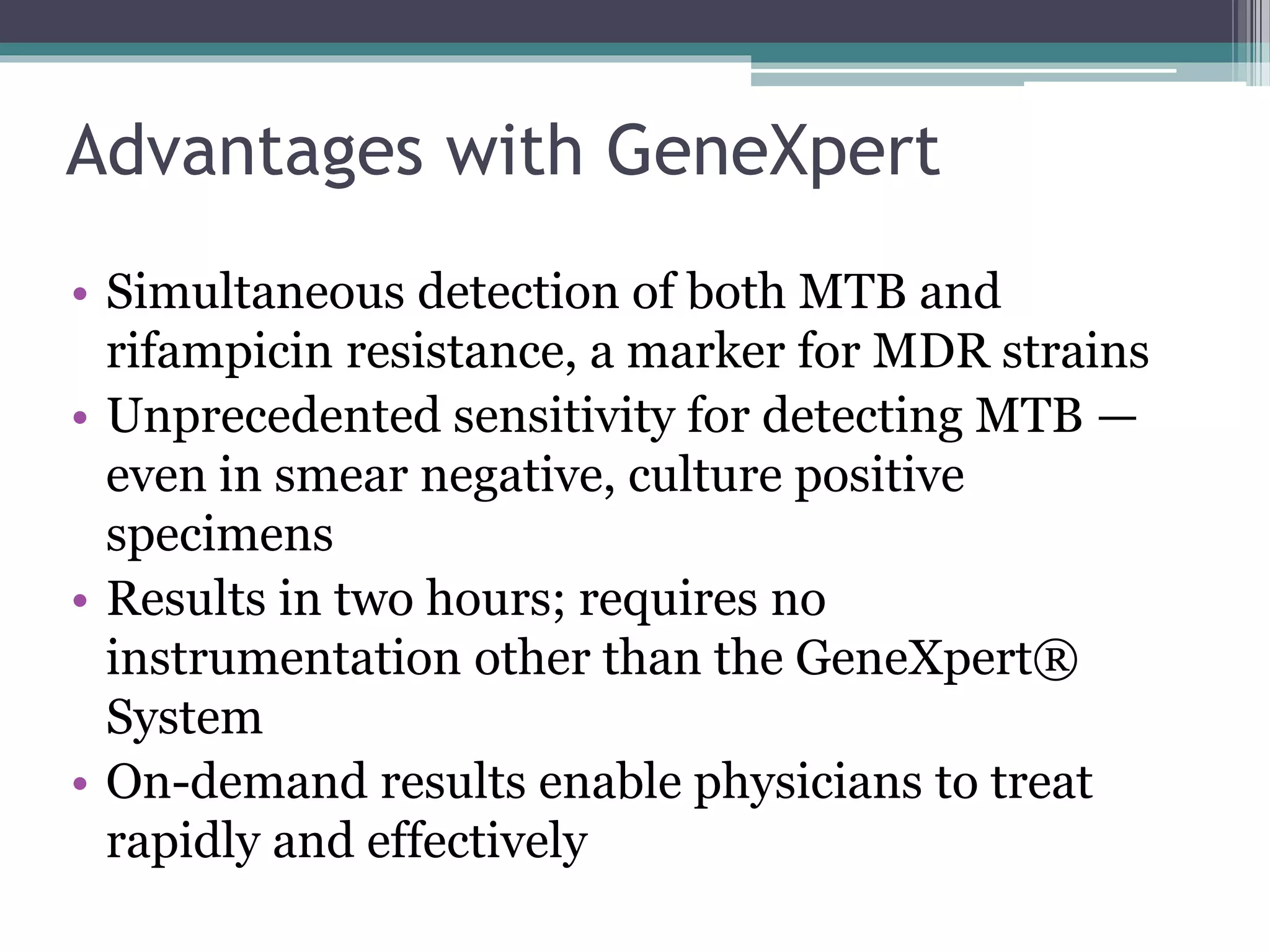
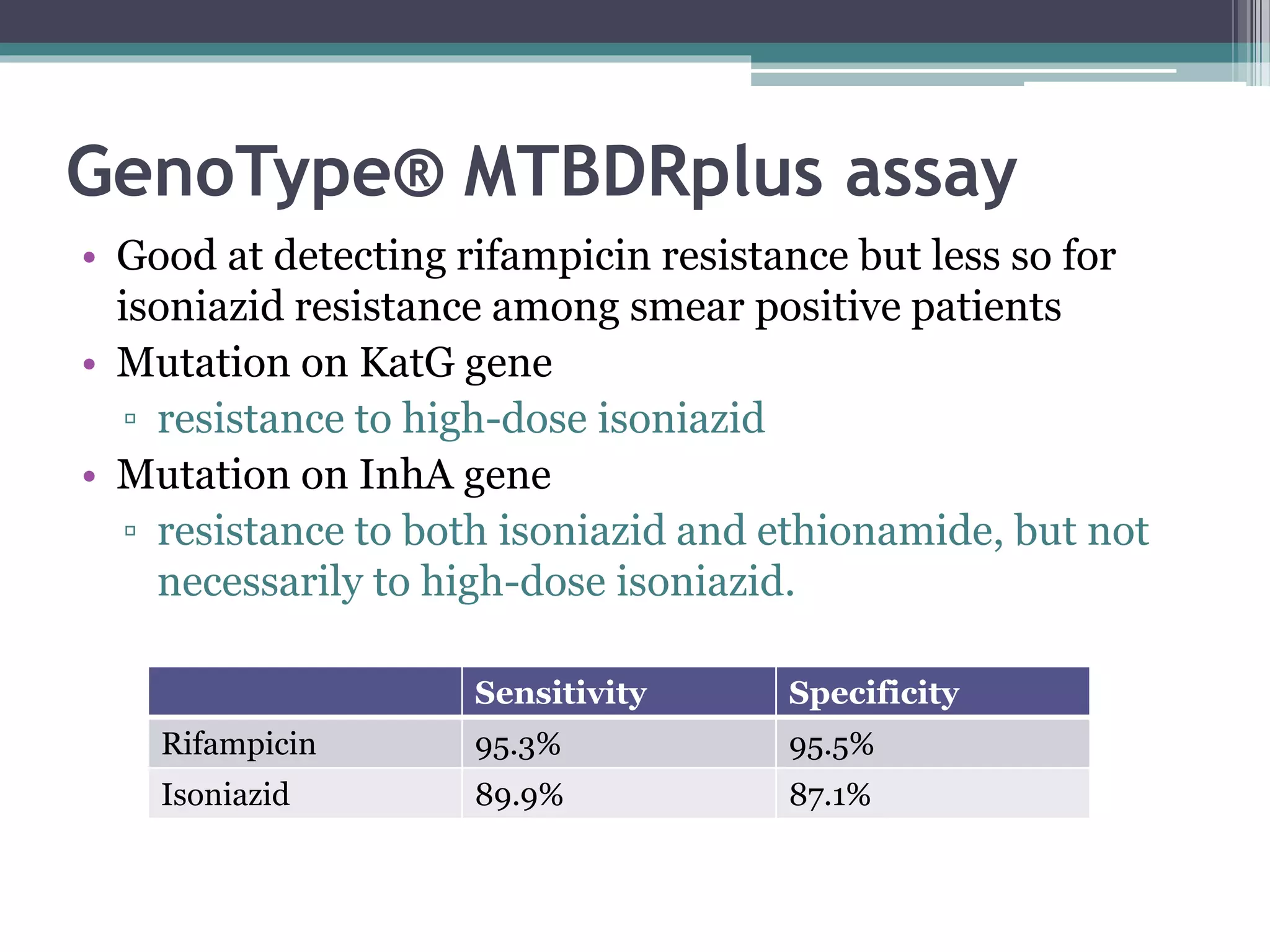
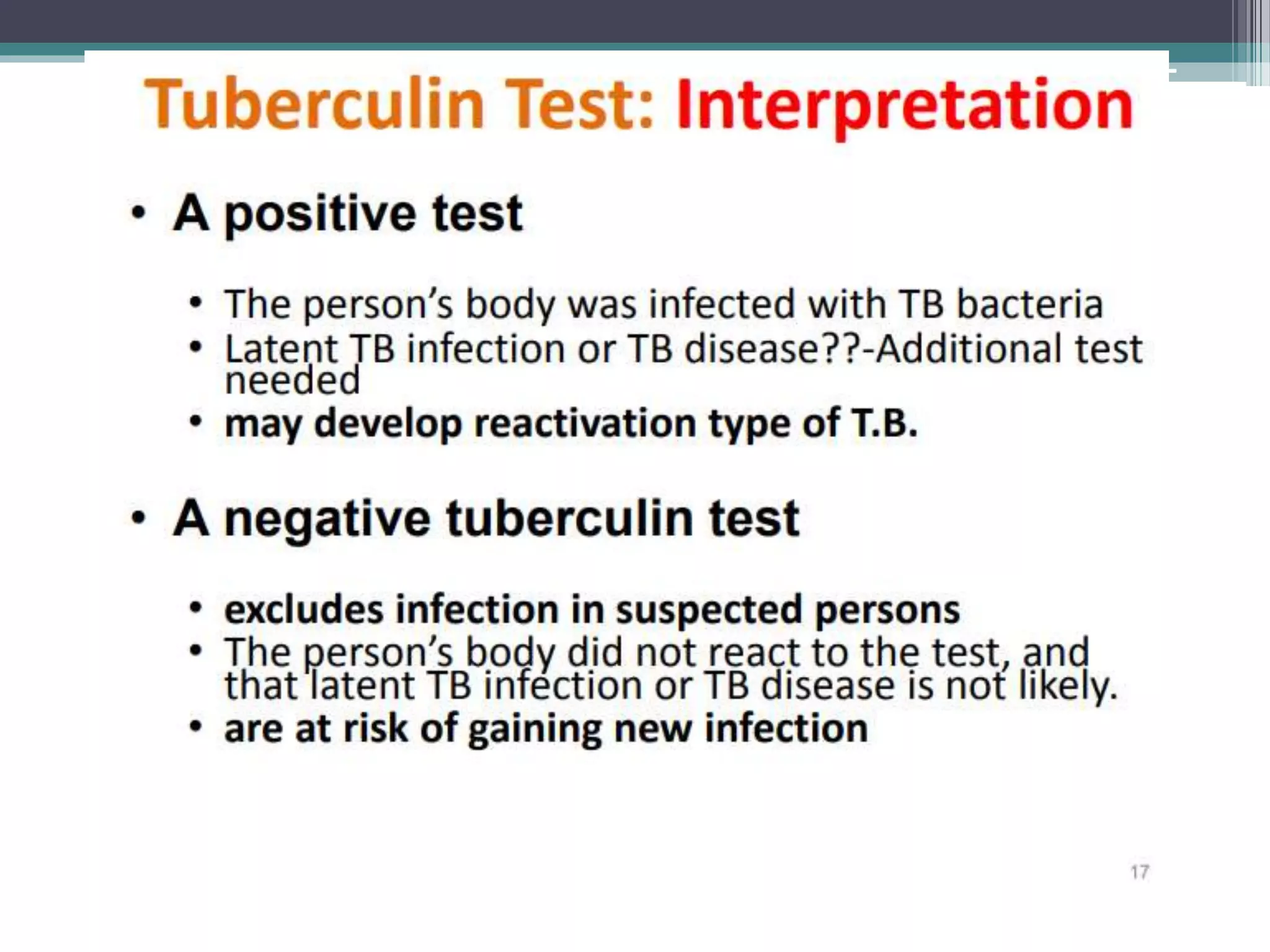
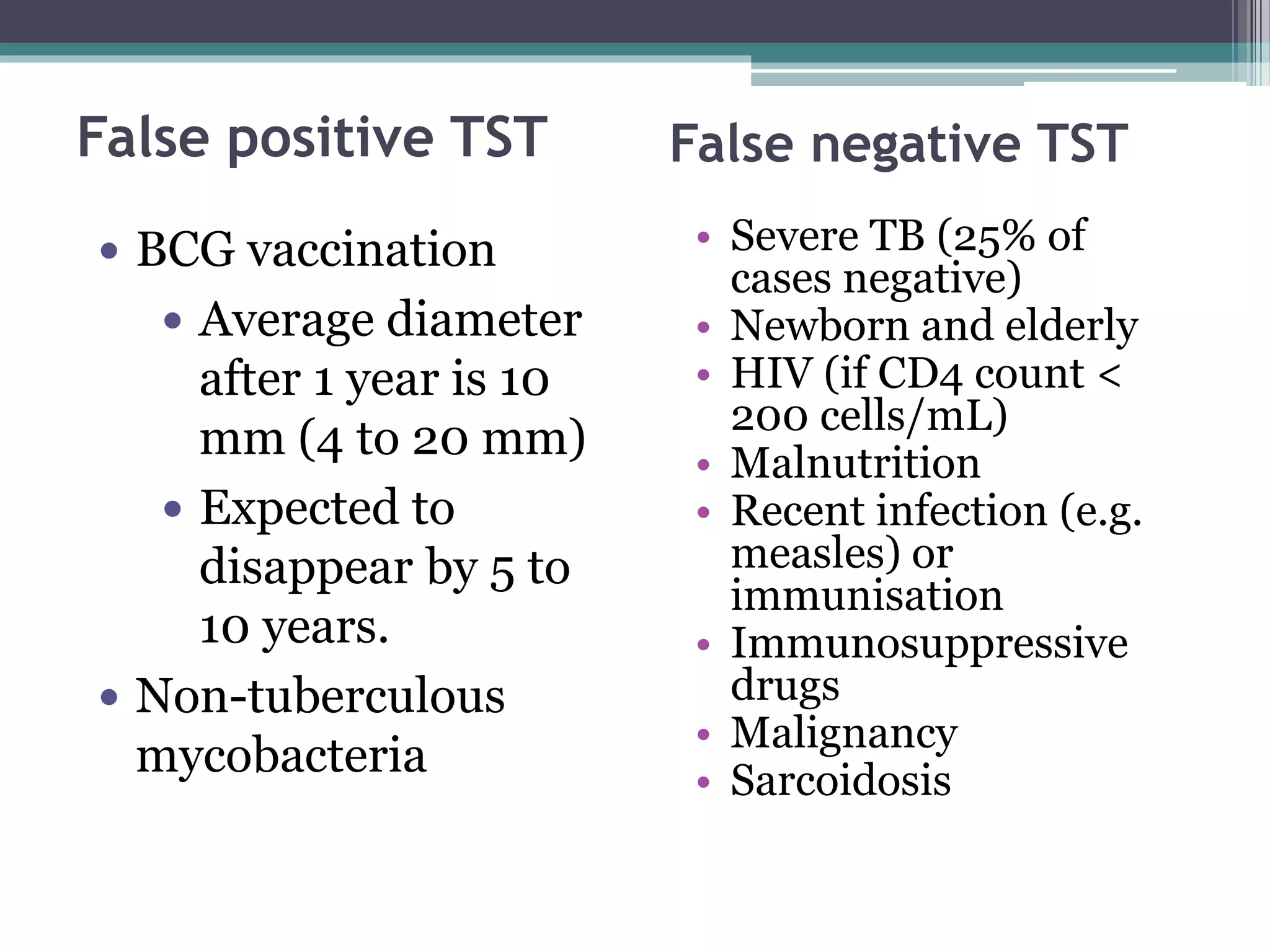
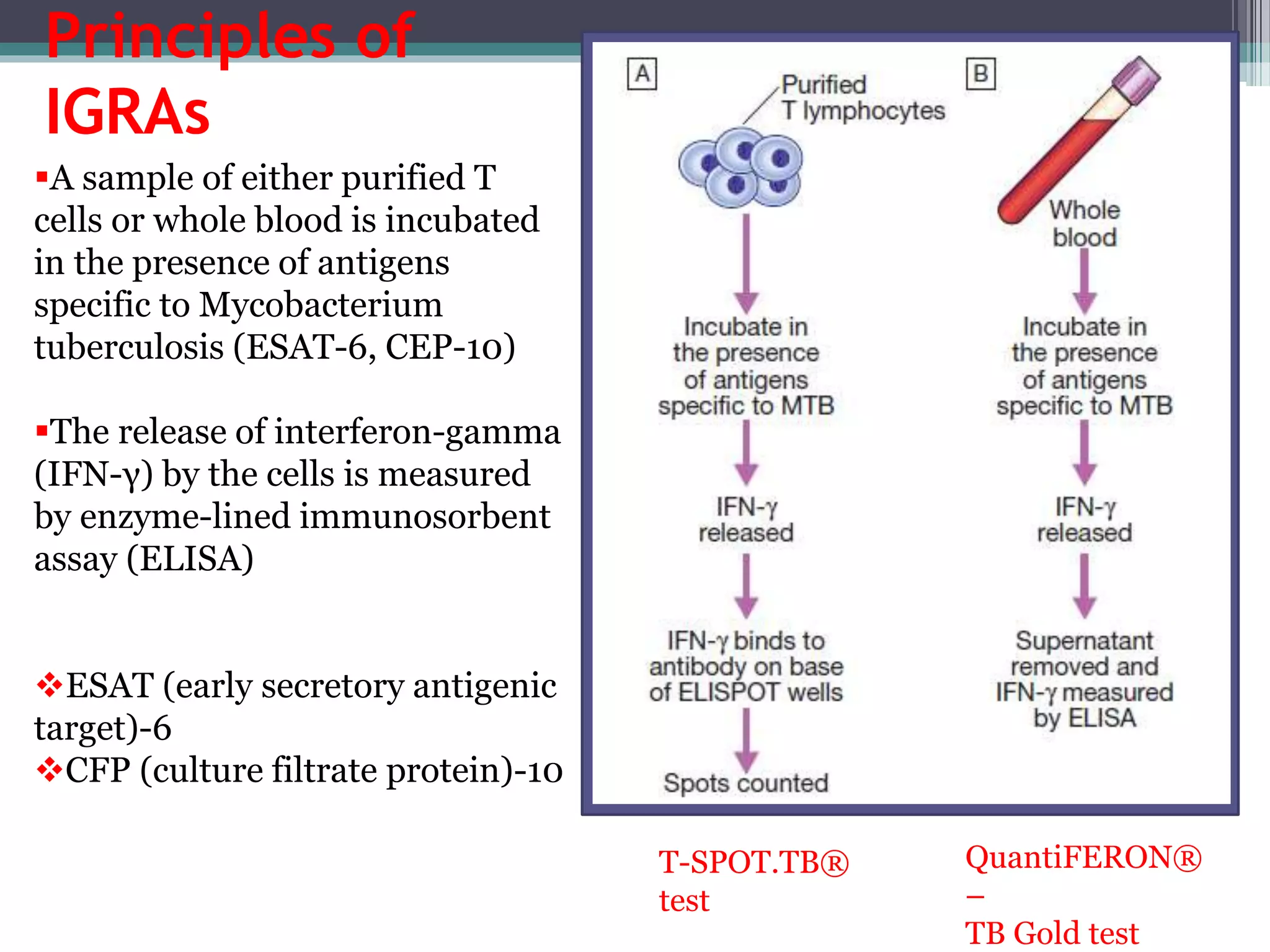
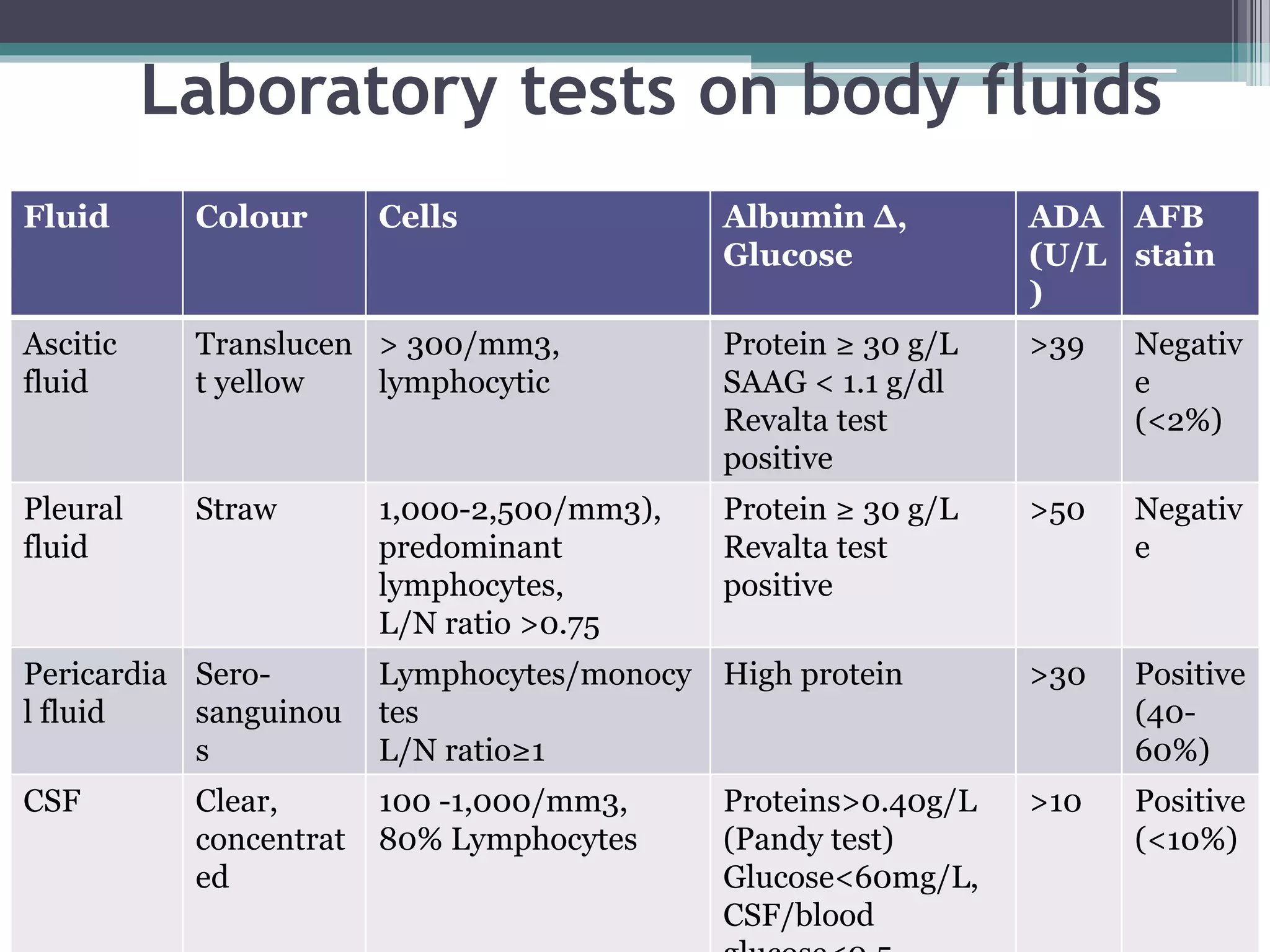
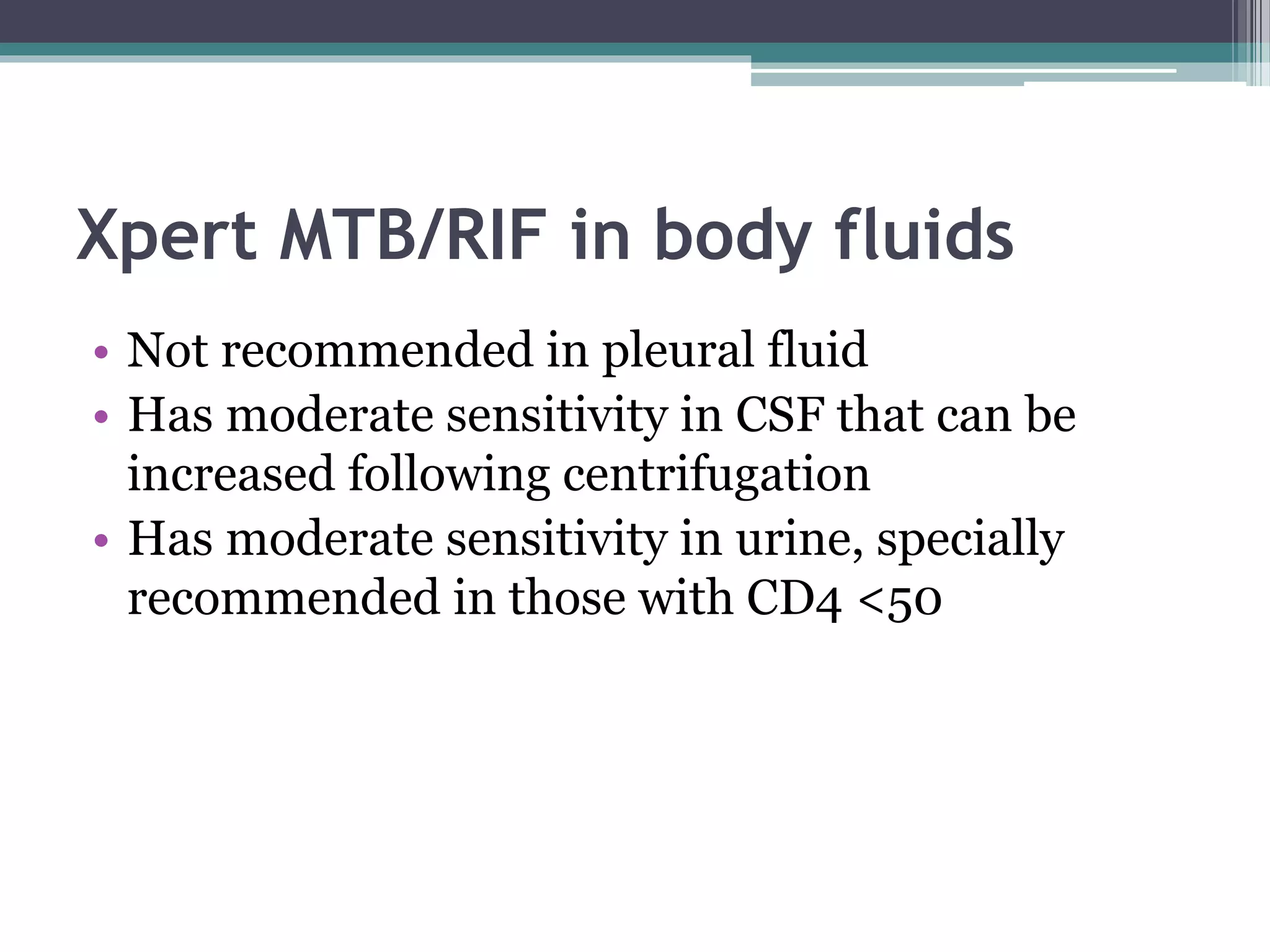
This document discusses various diagnostic modalities for pulmonary and extrapulmonary tuberculosis. It describes the characteristics of Mycobacterium tuberculosis and provides global burden statistics. It also discusses extra-pulmonary TB locations. Molecular techniques for diagnosis include Xpert MTB/RIF, which can simultaneously detect TB and rifampin resistance in under two hours. Other techniques discussed are line probe assays, drug susceptibility testing, radiology, tests for latent TB, and examinations of body fluids and biopsies. The document provides details on sensitivities and specificities of different diagnostic tests.