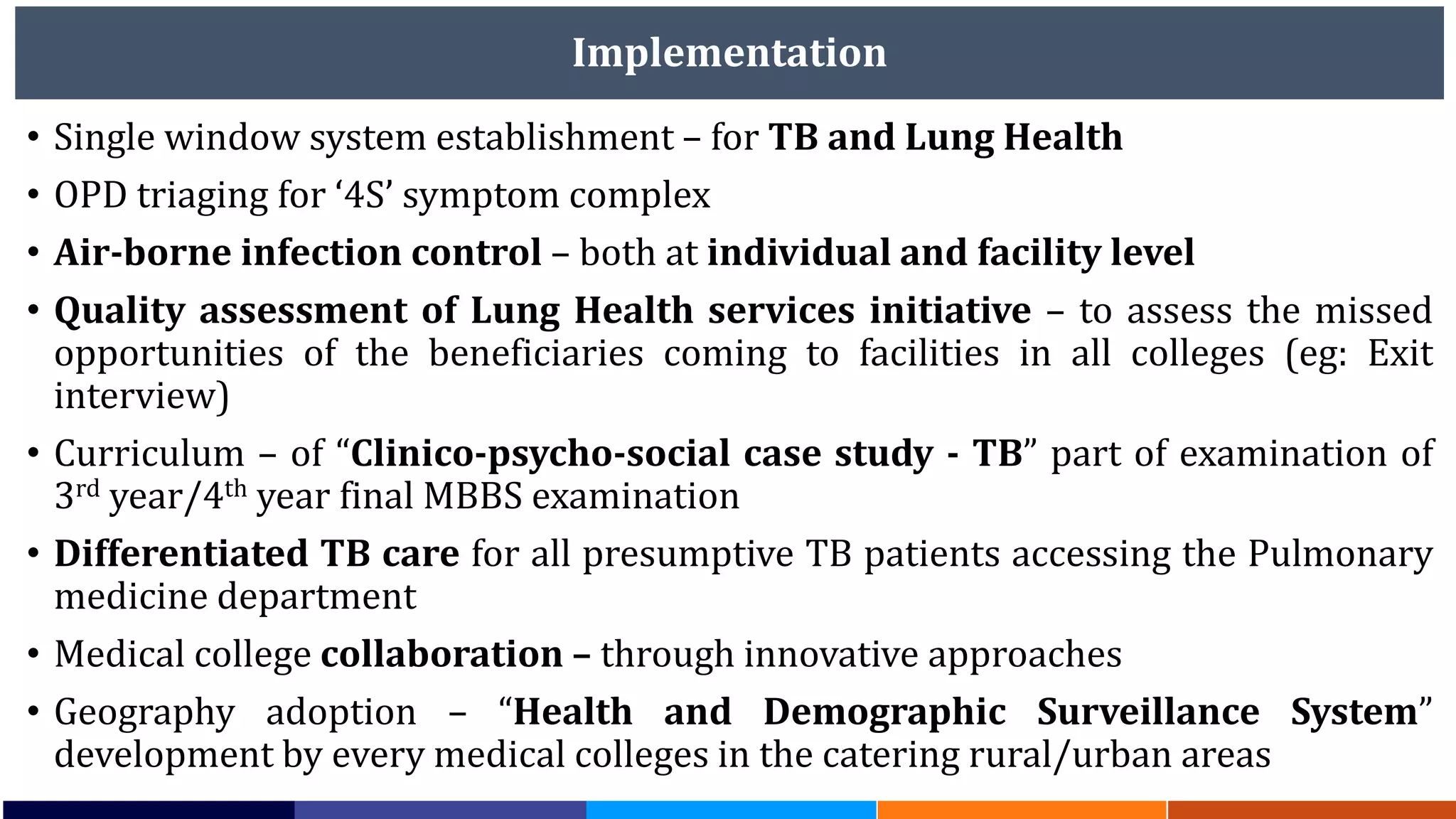
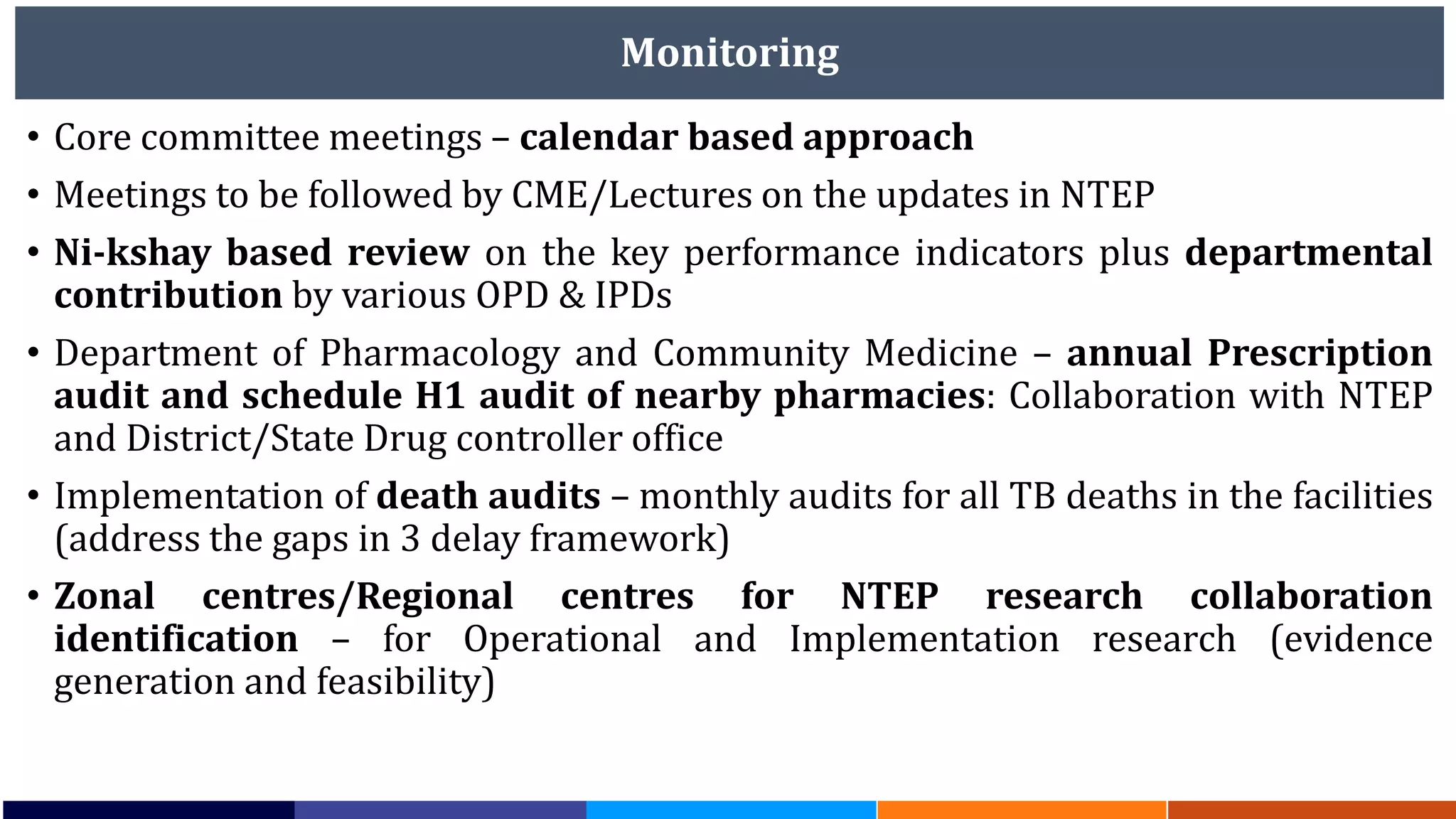
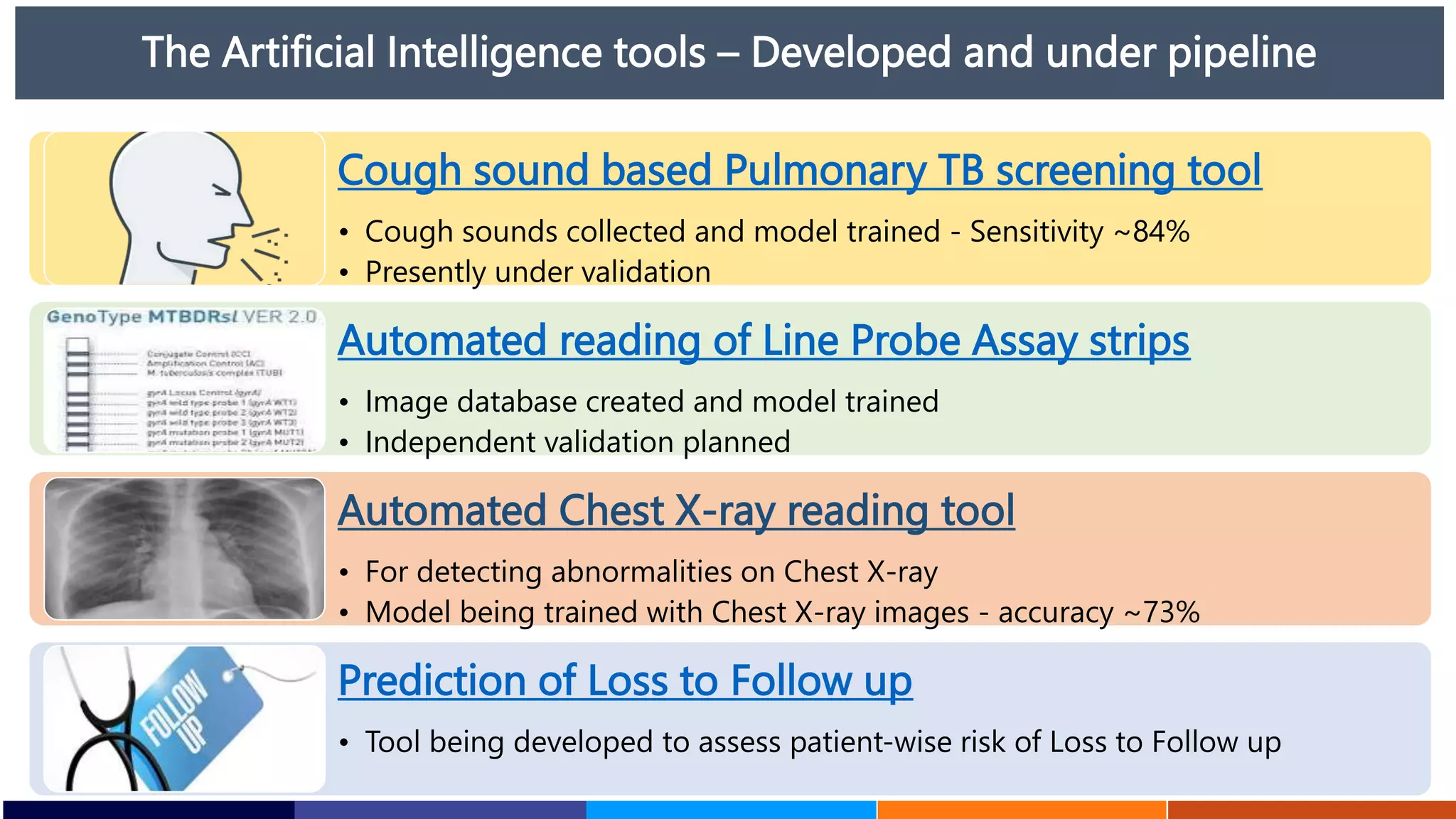
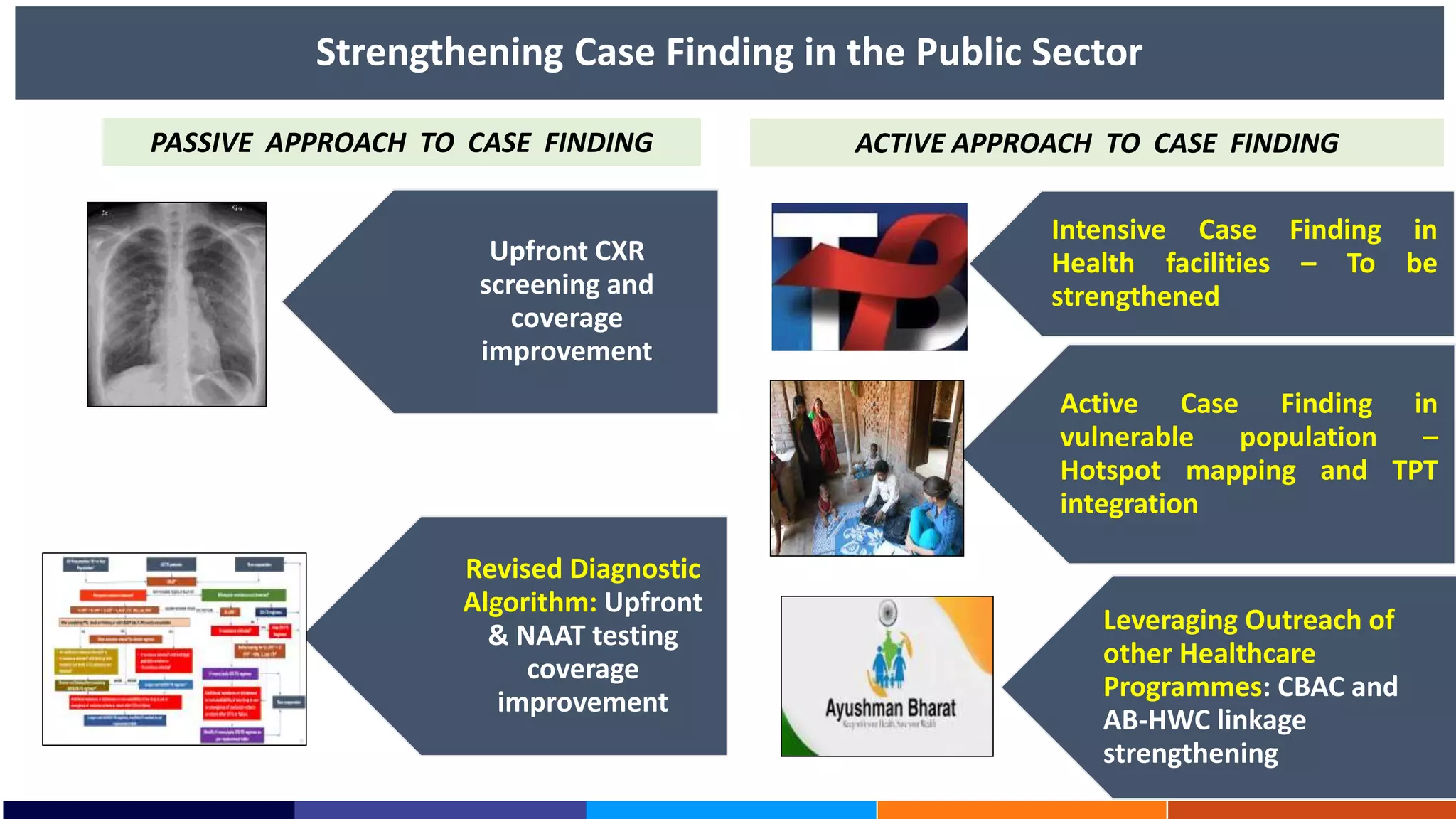
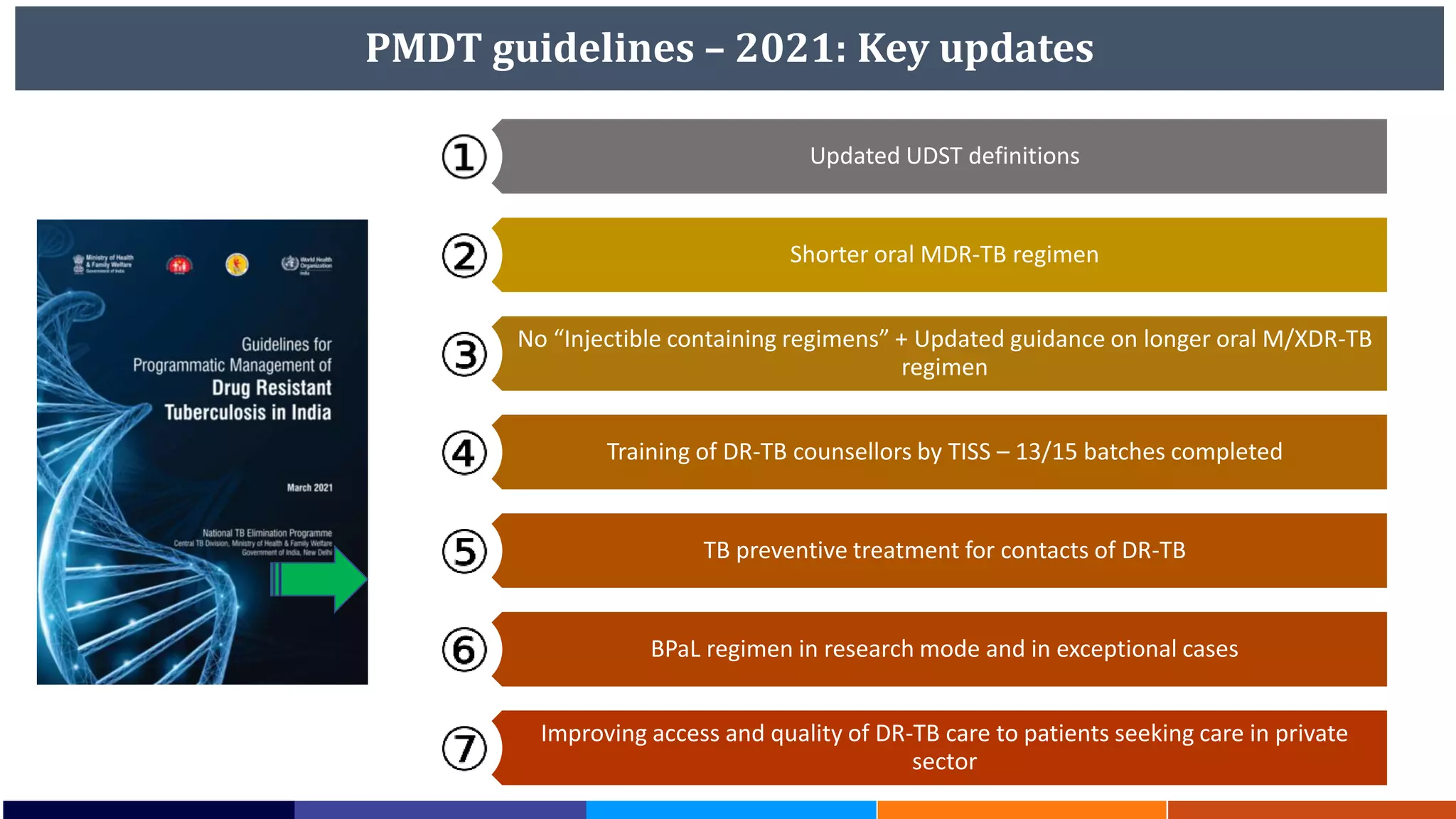
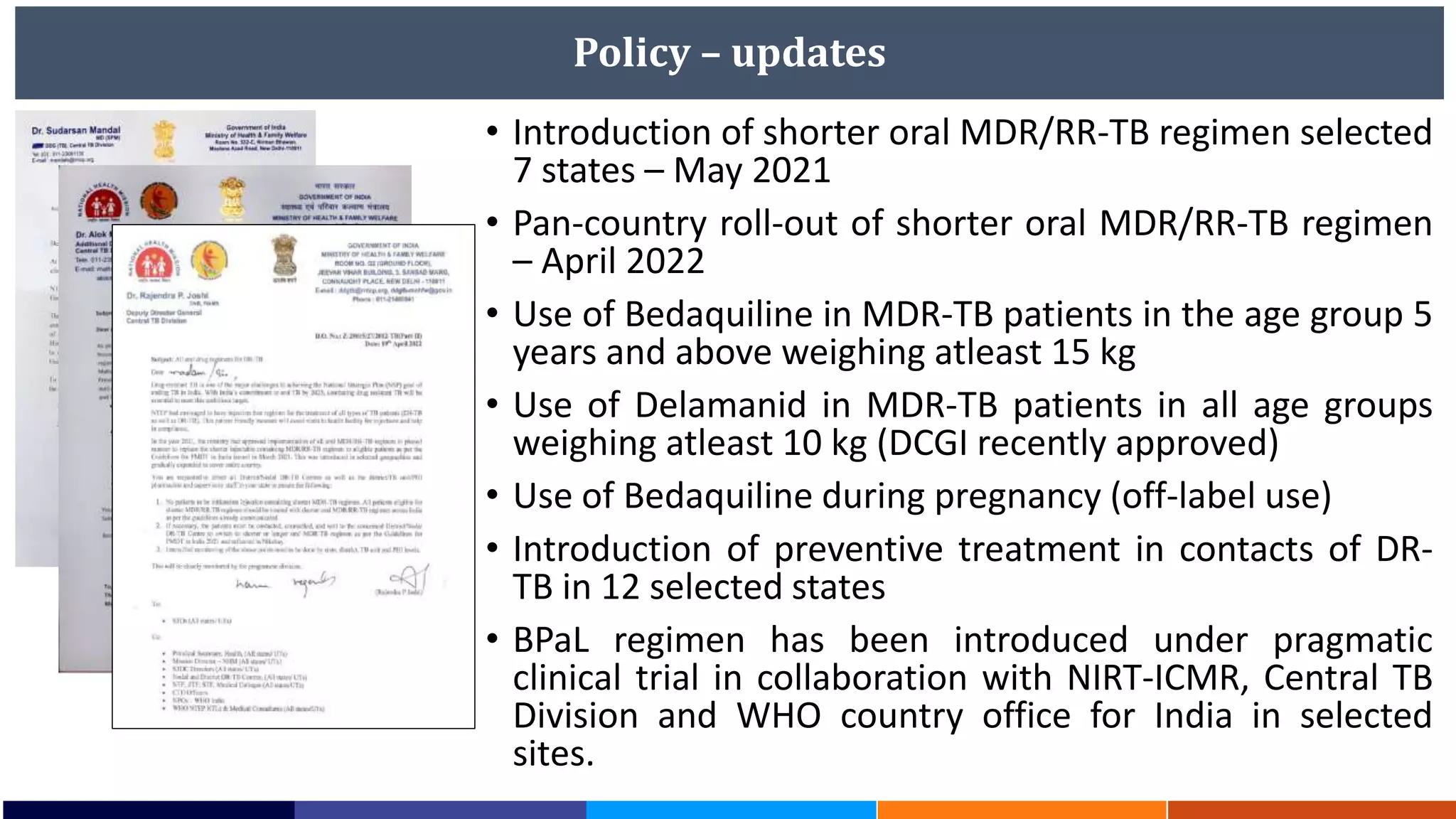
National Workshop for Medical Colleges Task Force to Accelerate Ending TB in India. The document outlines India's commitment to end TB by 2025, 5 years ahead of the global target. It discusses India's TB incidence and notification rates. It also summarizes the government's strategies like strengthening case finding, updated treatment guidelines, and new initiatives like the Subnational Certification for TB Free India program to bend the curve of the TB epidemic in India.










![Prevent - PMTPT policy related update
PMTPT policy Update
Target
population
Household contacts of pulmonary
TB*
Expansion of target population
PLHIV
NTEG meeting (23-24 Sep 2022): Malnourished,
Alcohol abusers, smokers and diabetics
Other risk groups - dialysis, silicosis,
initiated on Immunosuppresant or
anti-TNF, transplant recipients
NTEG meeting (23-24 Sep 2022): TPT intervention
to be merged with ACF and community level
intervention [next slide]
Testing
option
IGRA or TST
• IGRA or TST or Cy-TB
• DCGI approval for use of Cy-TB in age >/=18 years
• ICMR study is ongoing to validate feasibility of
Cy-TB in age <18 years [likely to get approval
from DCGI following study findings by year end]
Treatment
option
• 6H or 3HP
o 6H or 3HP
o 3RH expanded in 1+5 states
TPT in DR-
TB contact
• 6Lfx or 4R On-going and need to improve coverage](https://image.slidesharecdn.com/1-221206045923-604fb80b/75/NTEP-status-updates-and-plans-for-ending-TB-in-India-24-2048.jpg)