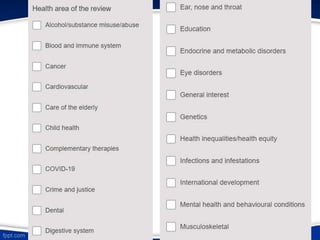
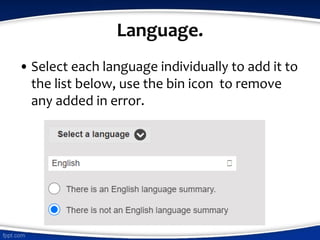
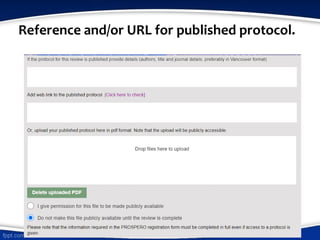
The document outlines guidelines for conducting systematic reviews, emphasizing the importance of a clear title utilizing the PI(E)COS structure, outlining expected start and completion dates, identifying the review team and their affiliations, and detailing potential conflicts of interest. It specifies procedures for defining review questions, participant criteria, interventions, and outcomes, along with search strategies and data extraction methods. Additionally, it discusses assessing risk of bias and strategies for data synthesis, providing examples to illustrate the required details for each component.