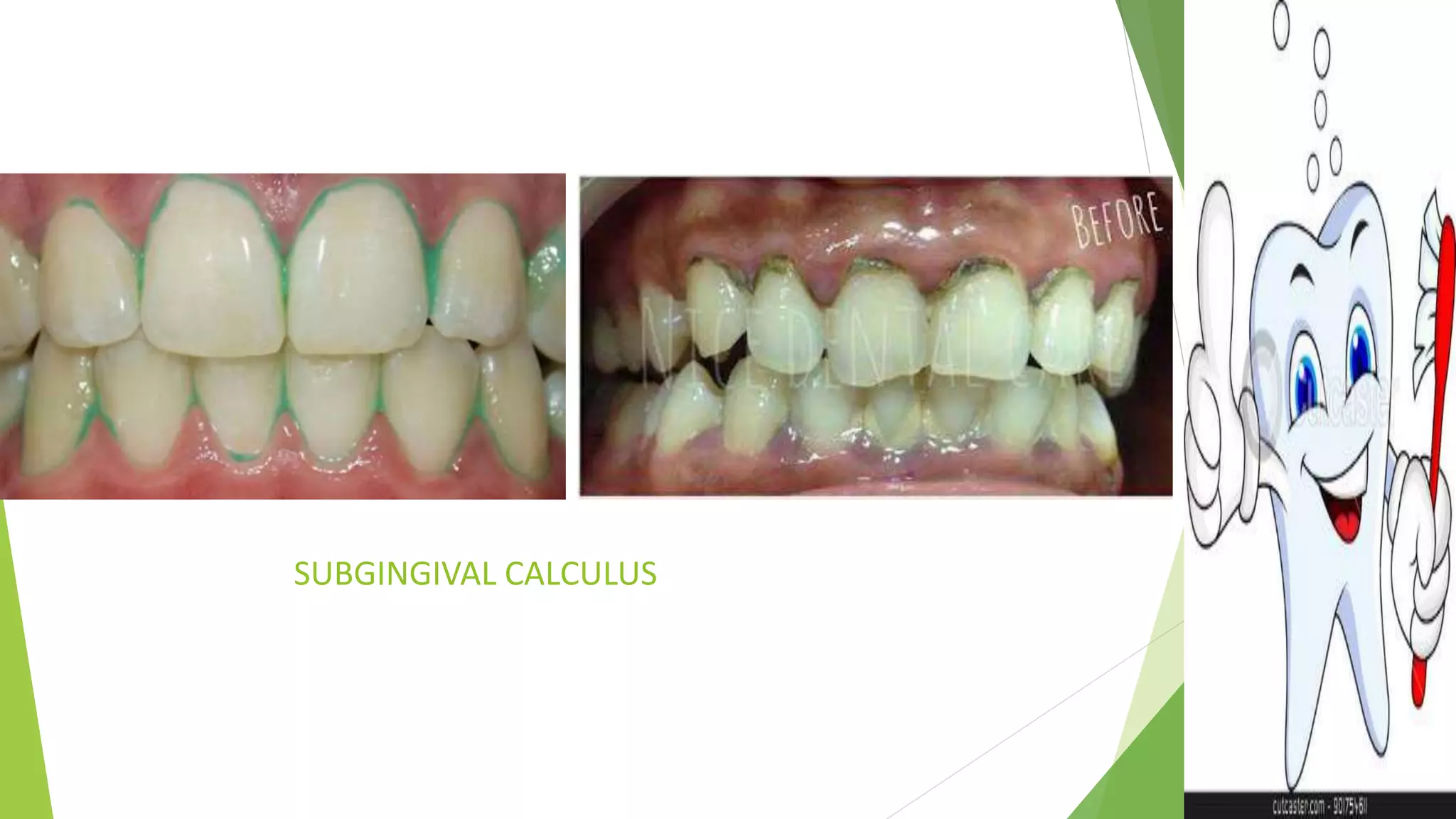
The document outlines dental calculus, including its definitions, classifications (supragingival and subgingival), and formation processes. It details the composition of calculus and the theories behind its formation, as well as various anticalculus agents and commercial products, such as chlorhexidine mouthwash. The information is presented in a structured format, highlighting the clinical significance of calculus in dentistry.