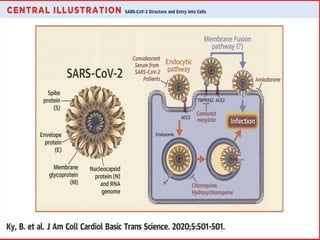
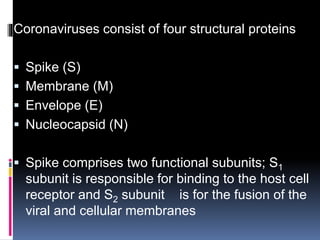
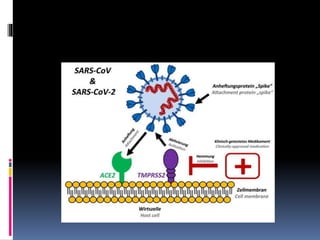
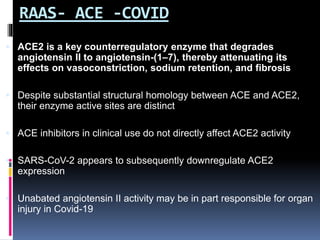
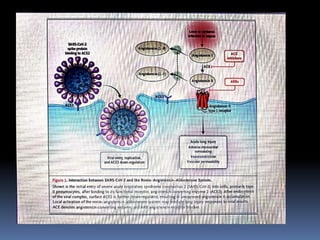
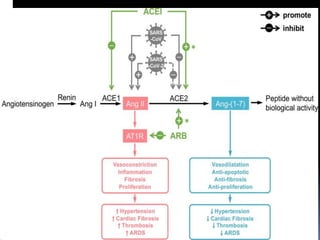
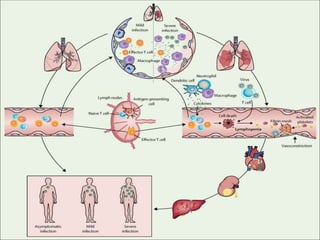
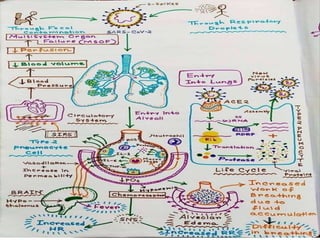
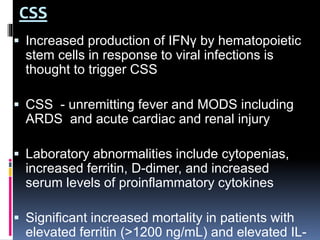
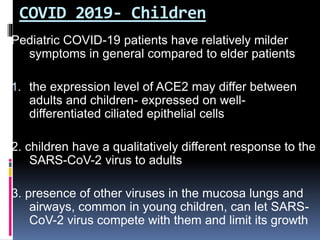
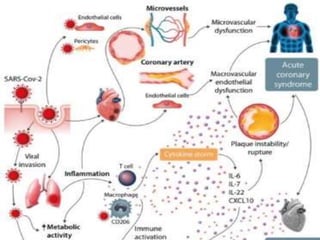
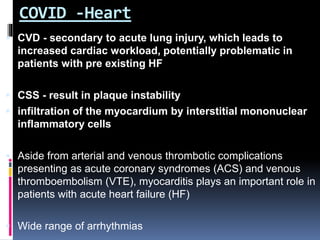
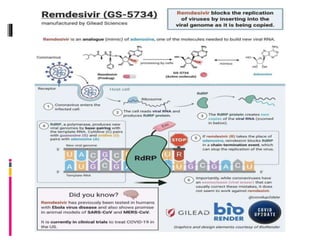
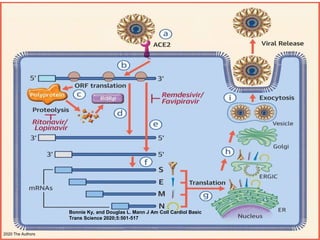
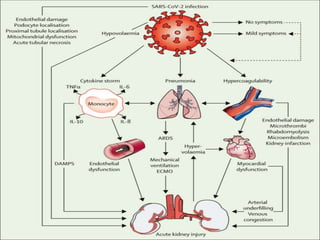
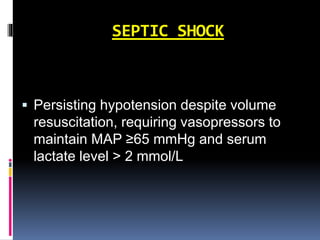
The document summarizes the pathophysiology of COVID-19. It discusses that SARS-CoV-2 enters cells through the ACE2 receptor and causes a cytokine storm. This can lead to organ damage and failure. Symptoms range from mild to severe and include fever, cough and shortness of breath. Those at highest risk are the elderly, immunocompromised, and those with pre-existing conditions like heart or lung disease. The clinical severity is classified as mild, moderate or severe based on symptoms and oxygen levels.