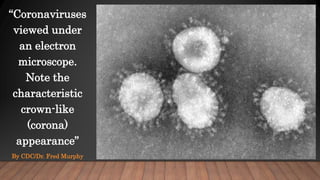
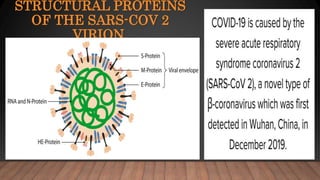
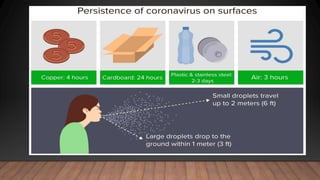
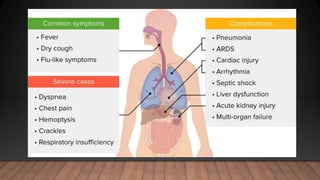
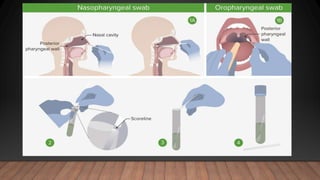
The document discusses COVID-19, its etiology as a coronavirus, transmission methods, clinical presentation, and epidemiology since its emergence in late 2019. It details the management, diagnostics, and investigational therapies for the disease, alongside prevention strategies and the status of vaccine development. As of March 2020, no specific treatment or FDA-approved vaccine was available, with ongoing clinical trials for potential vaccines and therapeutics.