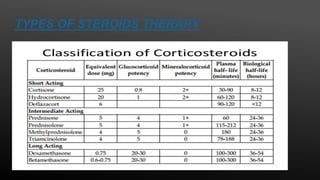
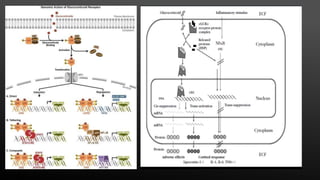
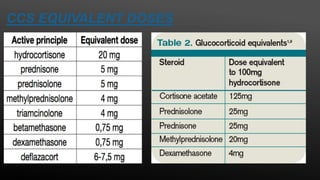
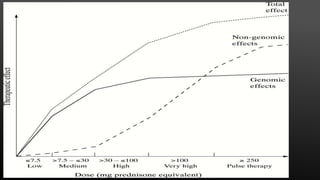
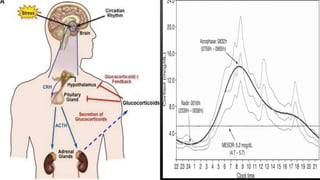
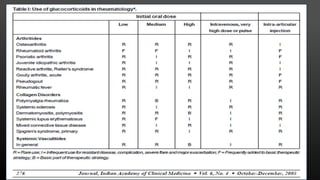
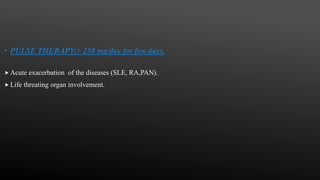
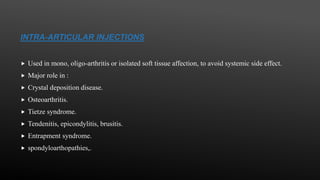
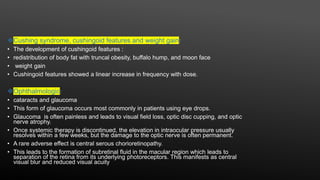
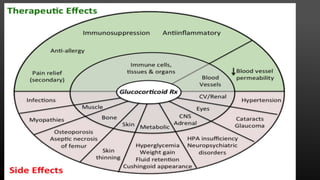
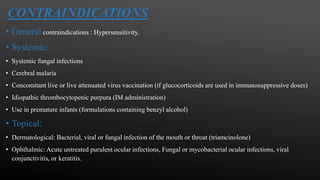
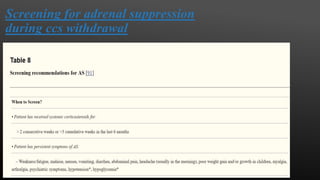
This document provides an overview of corticosteroid therapy. It discusses the types and doses of corticosteroids used, their mechanisms of action both genomic and non-genomic, indications for use, side effects, and guidelines for administration timing and withdrawal. Corticosteroids are powerful anti-inflammatory drugs that are widely used but also have numerous side effects, so their risks and benefits must be carefully considered.



![BRIEF HISTORY
1884, CCS were first used in patients with inflammatory arthritis
with dramatic result.
For 30 years, they have been used without any known mechanism
of both effect or adverse effect
. Tadeusz Reichstein, Edward Calvin Kendall. and Philip Showalter
Hench were awarded the Nobel Prize for Physiology and Medicine
in 1950 for their work on hormones of the adrenal cortex, which
culminated in the isolation of cortisone.
1955, high dose with short term GC was used and stabilized in RA.
Lewis Sarett of Merck & Co. was the first to synthesize cortisone,
using a 36-step process that started with deoxycholic acid, which
was extracted from ox bile.[45] The low efficiency of converting
deoxycholic acid into cortisone led to a cost of US $200 per gram..](https://image.slidesharecdn.com/corticosteroidtherapy-191202074628/85/Corticosteroid-therapy-4-320.jpg)