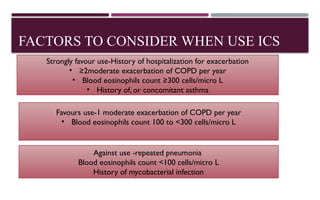
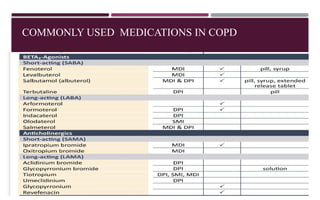
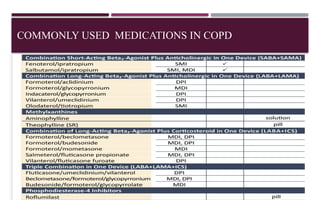
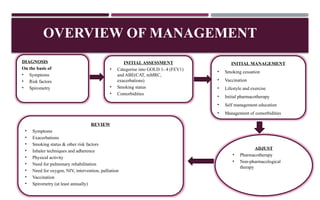
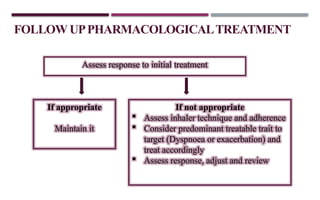
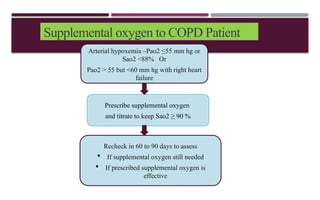
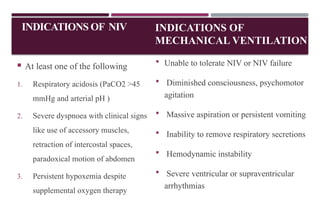
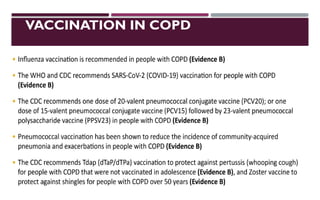
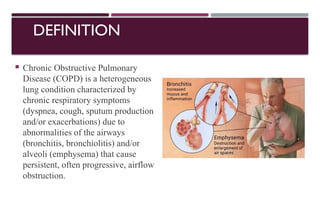
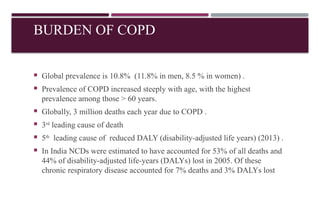
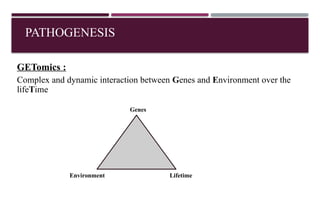
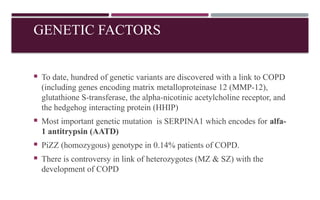
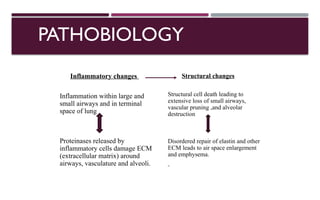
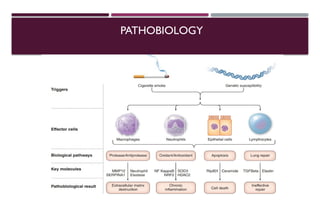
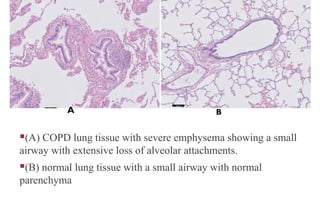
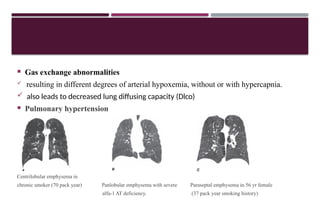
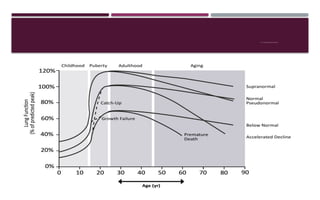
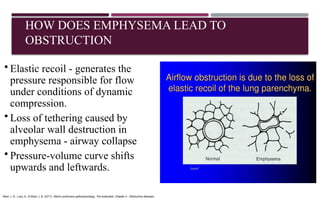
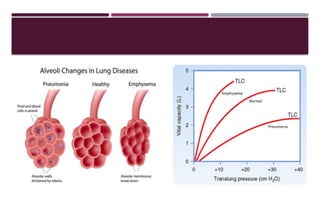
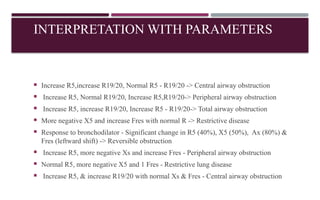
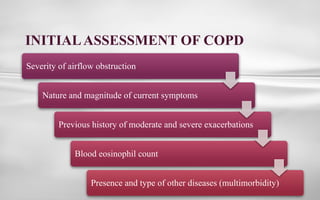
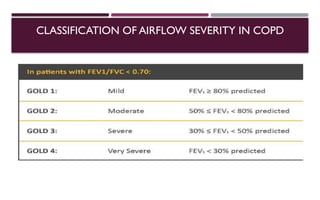
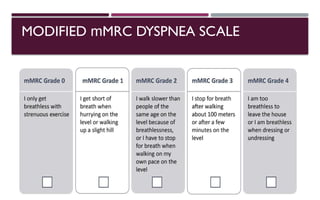
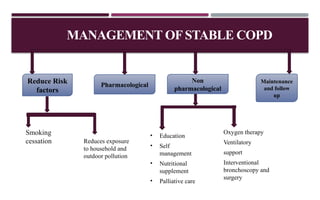
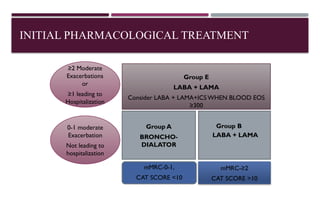
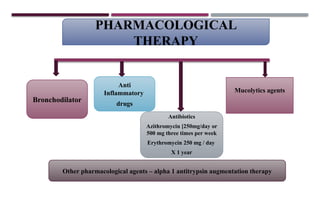
Chronic obstructive pulmonary disease (COPD) is a progressive lung condition characterized by airflow obstruction due to abnormalities in the airways and alveoli, affecting around 10.8% of the global population. It results in significant morbidity and mortality, with 3 million deaths each year, and is primarily linked to smoking, environmental factors, and genetic predisposition. The management of COPD includes pharmacological treatments, lifestyle modifications, and potential interventions to improve patient outcomes and quality of life.












![CLASSIFICATION OF EXACERBATION
Mild
[No respiratory failure]
Moderate
[Acute respiratory failure
- Non life threatening]
Severe
[Acute respiratory failure-
Life threatening]
Respiratory rate ≤24/min[20-30 /min] >24/min[>30 /min] >24/min[>30 /min]
Accessory muscle use Absent Present Present
Mental status Normal Normal Altered
Hypoxemia Improved with supplemental
oxygen (FiO2: 24-35 % )
Improved with supplemental
oxygen
(FiO2 > 35 % )
Not improved even with
supplemental oxygen
(FiO2 > 40 % )
PaCO2 Normal 50-60 mmHg >60 mmHg (pH < 7.2)](https://image.slidesharecdn.com/copdfinal2-240915142029-7db9b9fe/85/COPD-DEFINITION-PATHOPHYSIOLOGY-AND-MANAGEMENT-40-320.jpg)