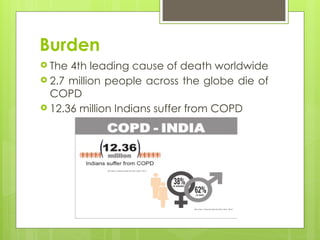
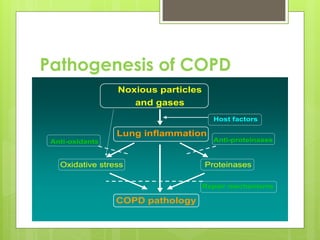
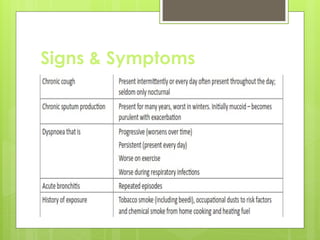
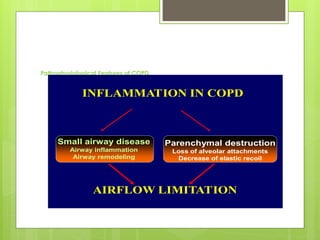
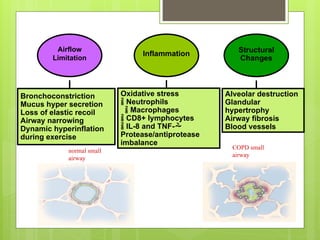
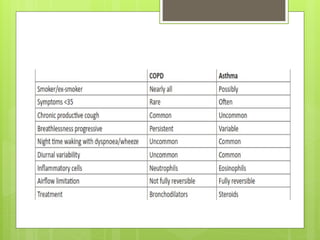
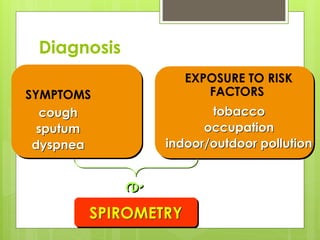
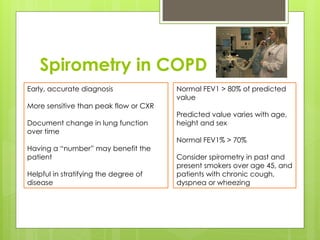
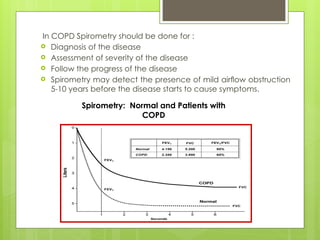
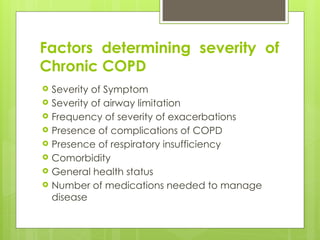
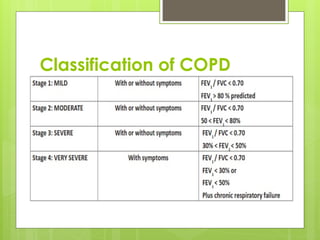
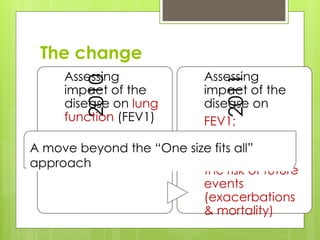
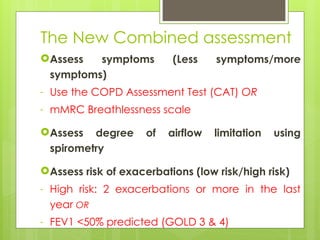
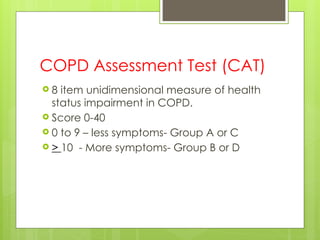
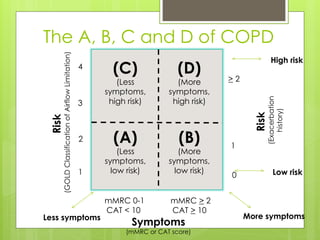
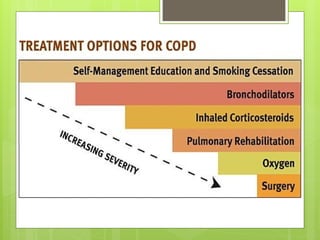
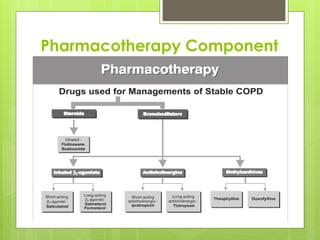
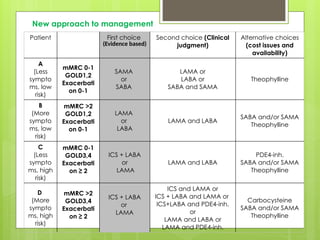
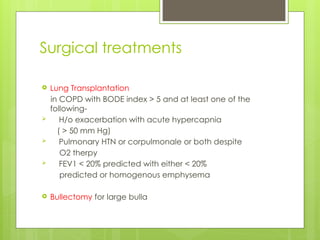
Chronic Obstructive Pulmonary Disease (COPD) is a preventable and treatable condition characterized by persistent airflow limitation largely caused by smoking, with significant impacts on systemic health. The disease spectrum includes chronic bronchitis, emphysema, and asthma and is typically diagnosed through spirometry, which also assesses severity and progression. Management integrates pharmacotherapy, non-pharmacological approaches, and addressing exacerbations to improve patient outcomes and quality of life.