The document discusses the structure and properties of water molecules. It explains that a water molecule is made up of two hydrogen atoms bonded to one oxygen atom at an angle of 105 degrees, giving the molecule a slight positive and negative charge. This allows hydrogen bonds to form between water molecules, giving water its unique liquid properties even at room temperature. The document also describes how water can absorb large amounts of heat without much change in temperature, and how its heat capacity helps regulate temperatures in the environment.







![Water is a very unusual compound; it is
very common and is found in all three
conditional states, solid (as ice), liquid (as
water) and gas (as water vapor). Other types
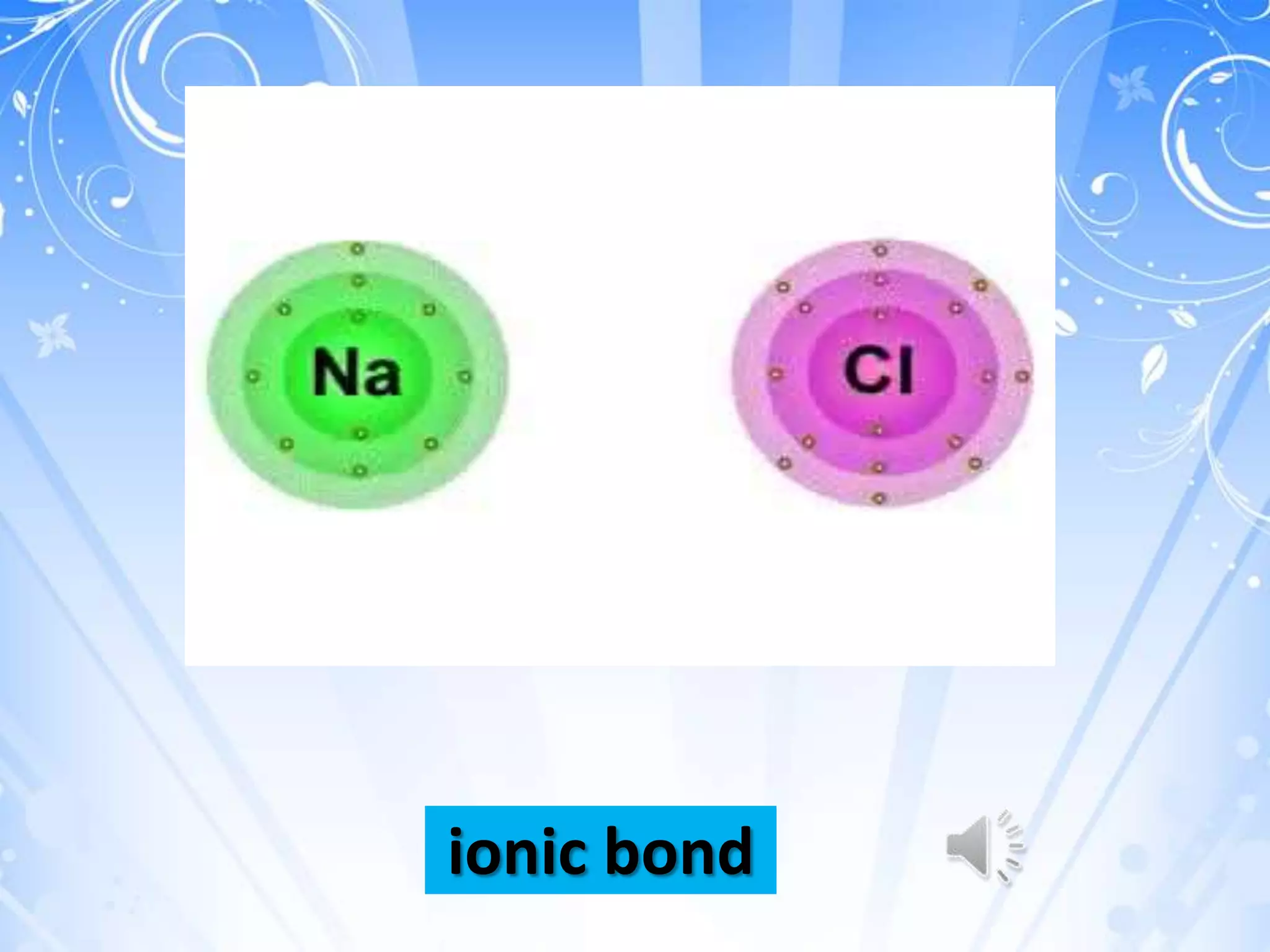
of bonding can occur, such as covalent
bonding (as seen in the formation of
molecular oxygen) or ionic bonding (as seen in
the formation of salt or sodium
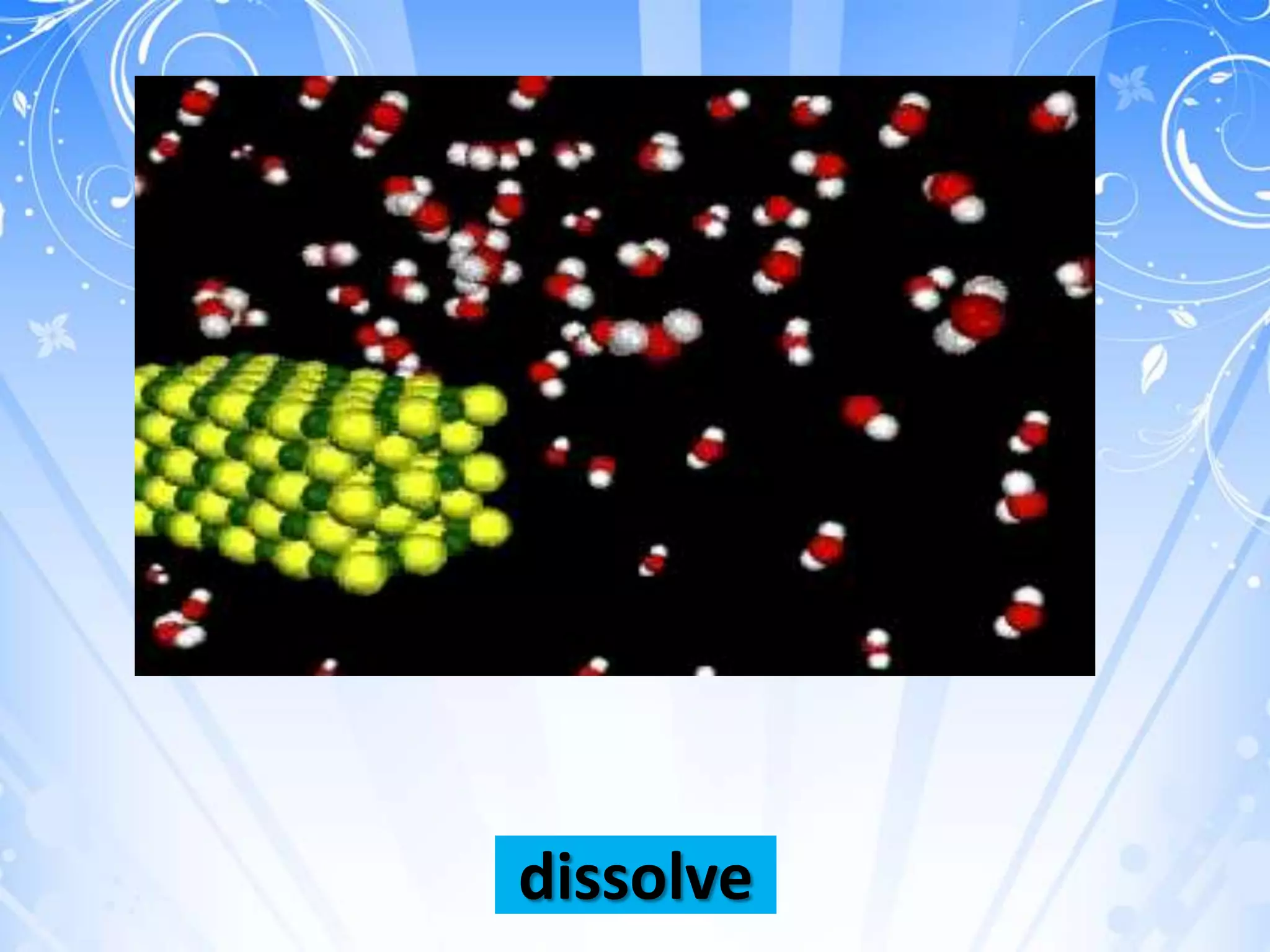
chloride[NaCl]). Hydrogen bonding can break
up the electrical attraction of atoms of solids
and dissolves them.](https://image.slidesharecdn.com/cooperativelearning-130205100639-phpapp01/75/Cooperative-learning-13-2048.jpg)