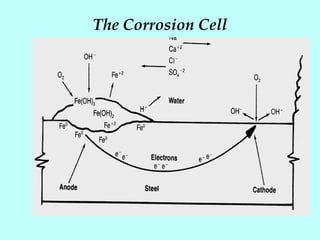
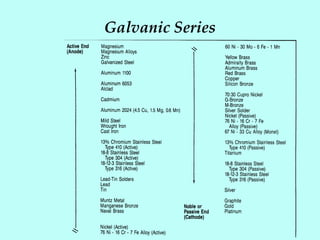
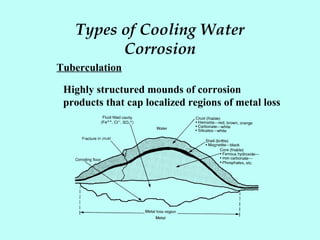
This document discusses corrosion in cooling water systems. It outlines the corrosion process and the elements required for corrosion to occur. The rate and type of corrosion are determined by factors at the cathode and anode. Various types of corrosion in cooling water systems are described, including general etch, concentration cell corrosion, cracking, and mechanical damage. Finally, general methods for corrosion inhibition are presented, such as using corrosion resistant materials, coatings, cathodic protection, water chemistry adjustments, and corrosion inhibitors.