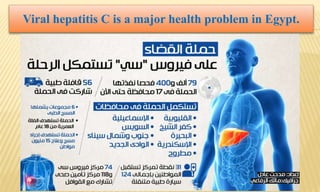
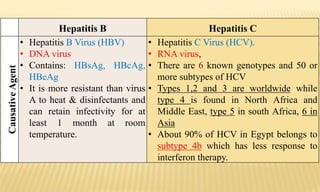
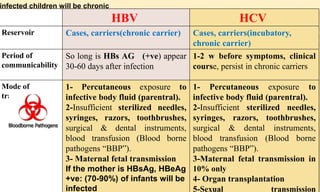
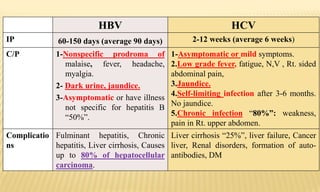
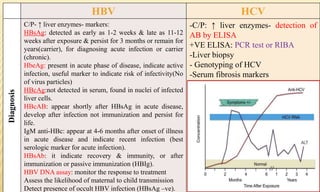
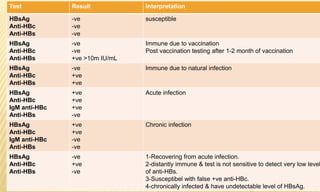
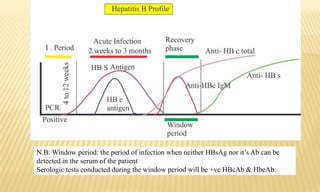
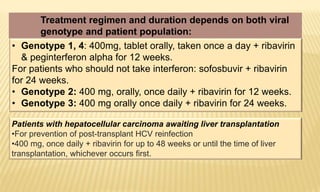
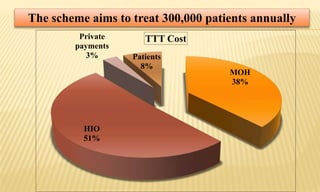
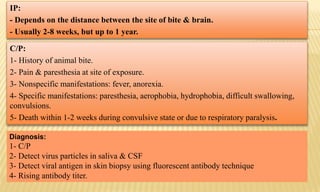
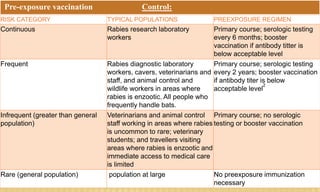
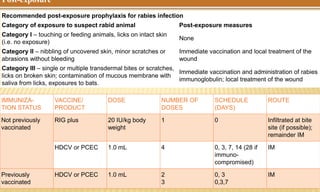
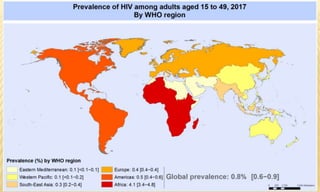
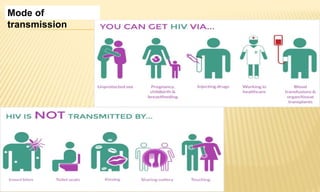
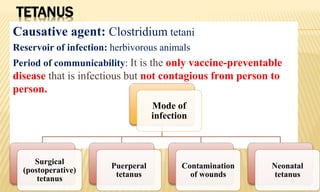
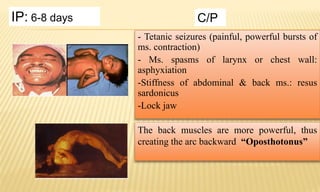
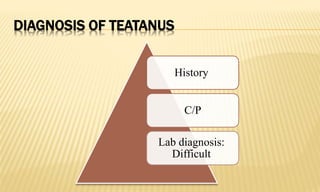
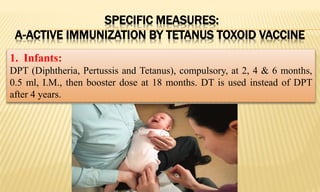
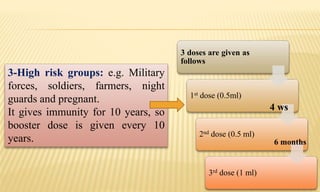
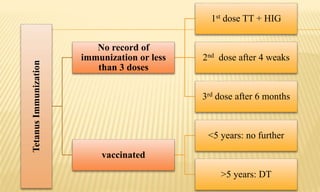
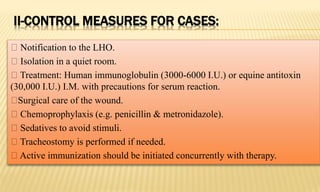
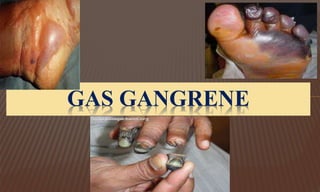
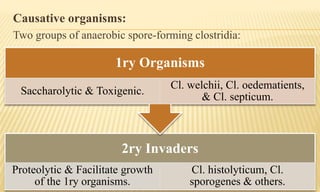
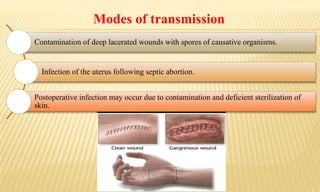
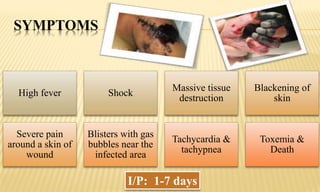
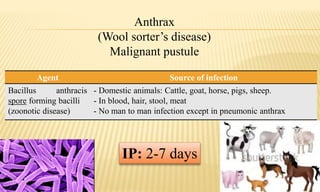
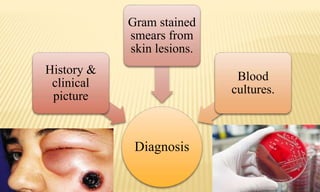
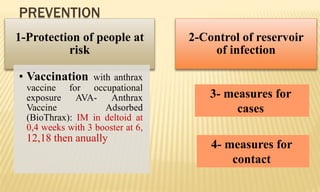
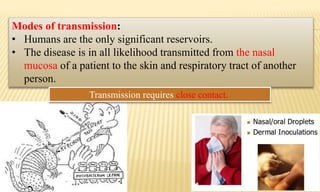
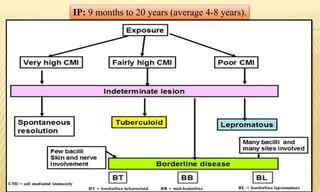
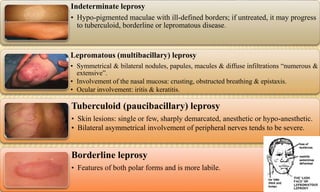
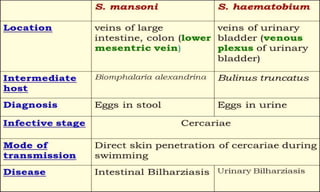
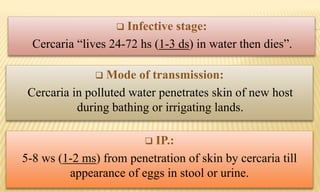
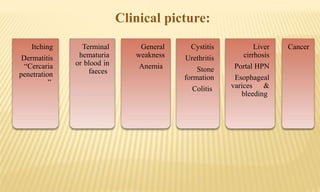
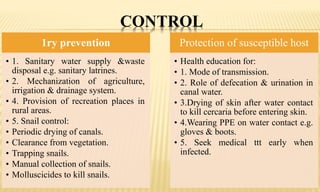
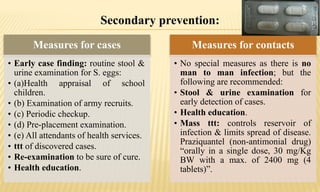
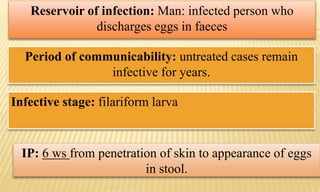
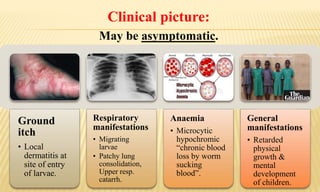
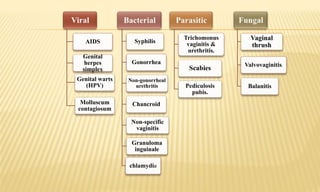
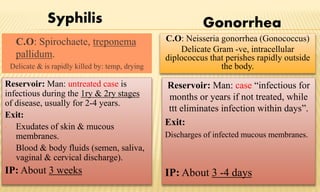
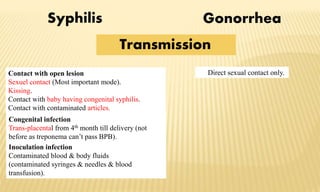
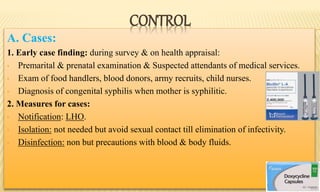
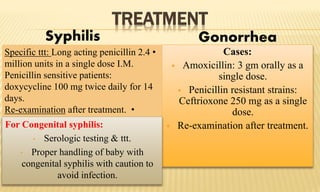
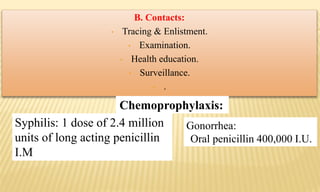
This document provides information on contact infections, including viral hepatitis C which is a major health problem in Egypt. It discusses the causative agents, modes of transmission, symptoms, diagnosis and treatment of viral hepatitis B and C. It also provides details on Egypt's national strategy to control the hepatitis C epidemic through widespread treatment programs aiming to reduce the prevalence of chronic infection.