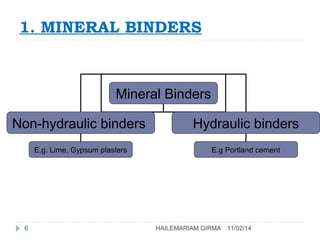
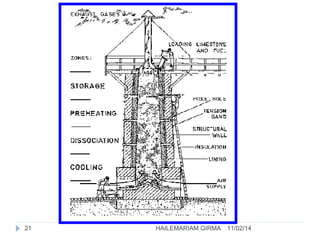
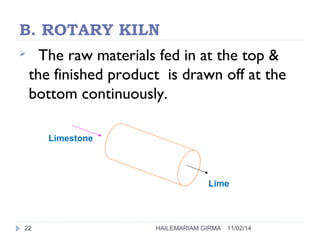
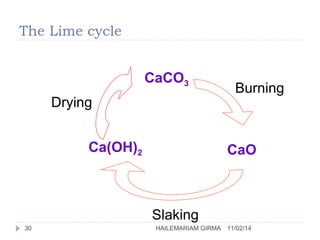
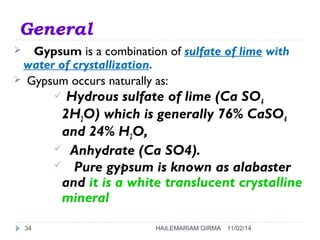
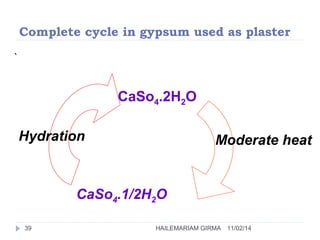
Binders are substances that are used to bind particles and fibers to form strong components. There are three main groups of binders: mineral, bituminous, and synthetic. Mineral binders include lime and gypsum plasters, which are non-hydraulic, and Portland cement, which is hydraulic. Lime is produced by burning limestone and is used as quicklime, hydrated lime, or hydraulic lime. Gypsum plasters include plaster of Paris and hard-finish plaster, which are produced by heating gypsum at different temperatures.