Embed presentation
Download to read offline
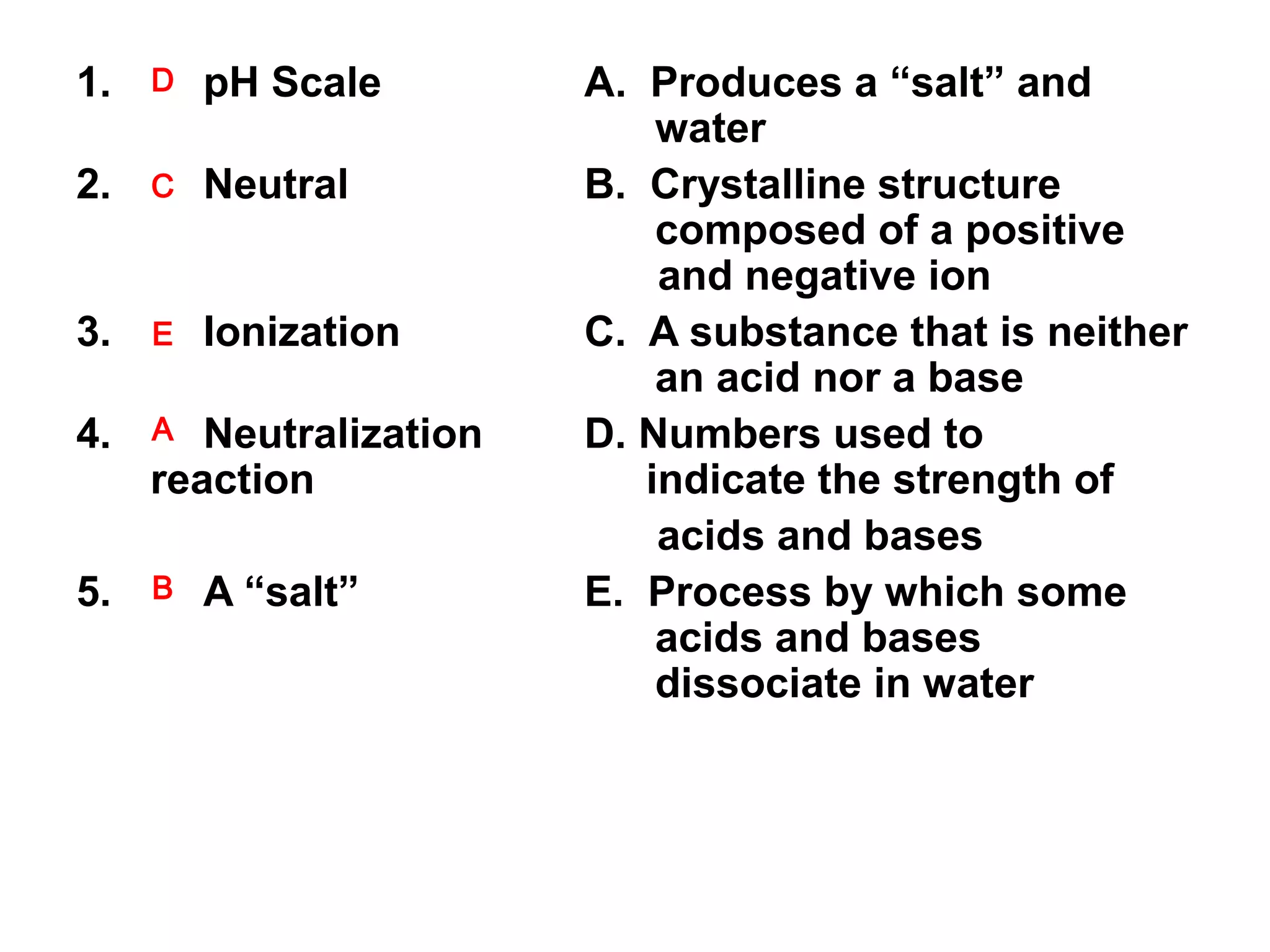



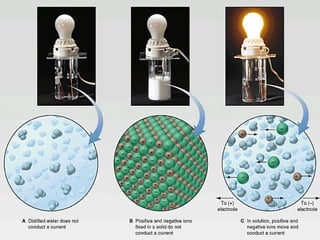


This document discusses conductivity of solutions. When acids, bases, or salts dissolve in water, their atoms separate and become charged ions that can conduct electricity. Solutions of acids, bases, and salts are conductive because they contain ions, while solutions of substances like alcohol, sugar, or distilled water are non-conductive because their atoms do not separate into ions. Conductivity occurs when dissolved substances ionize into charged particles in water.






