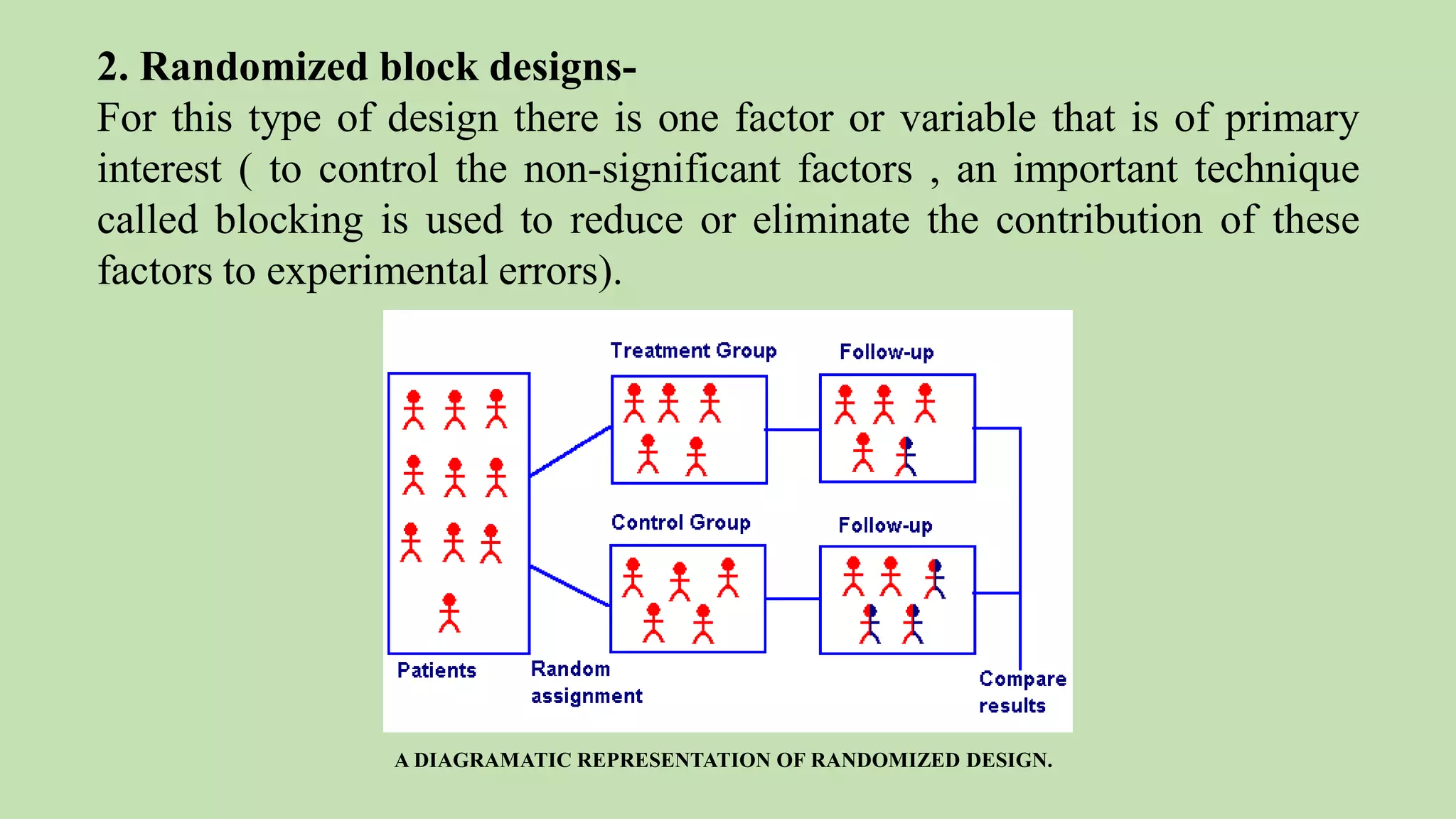
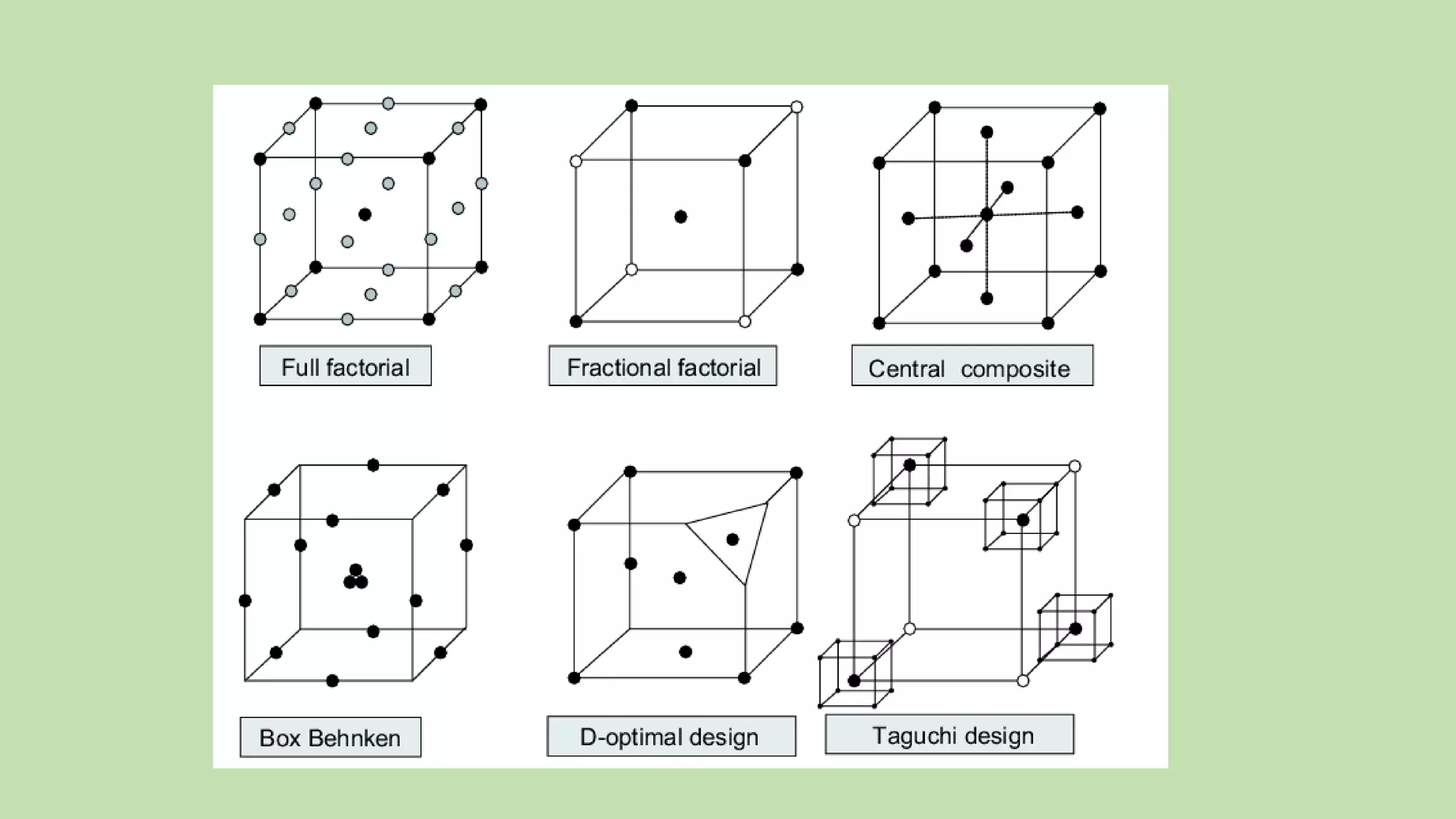
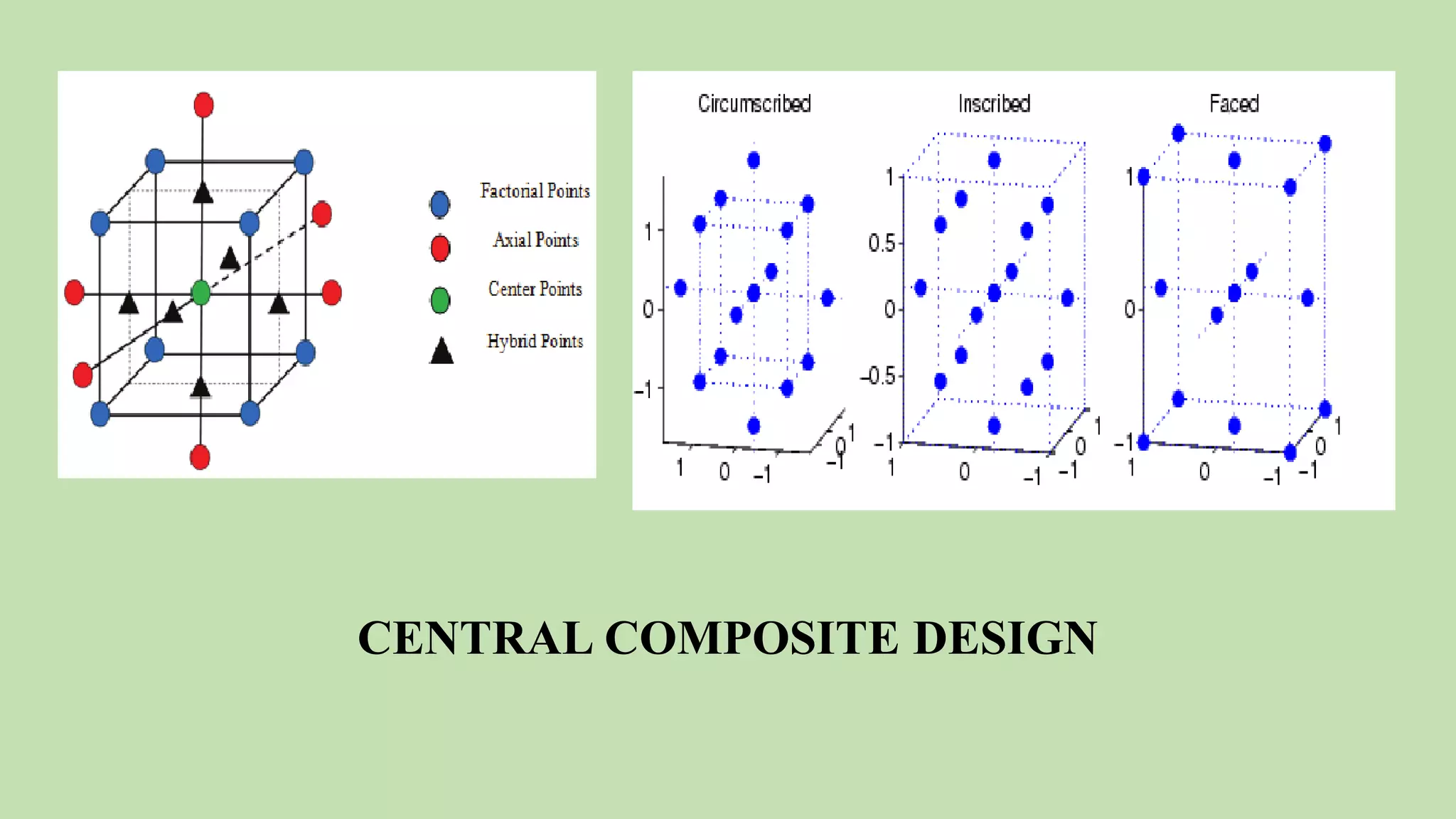
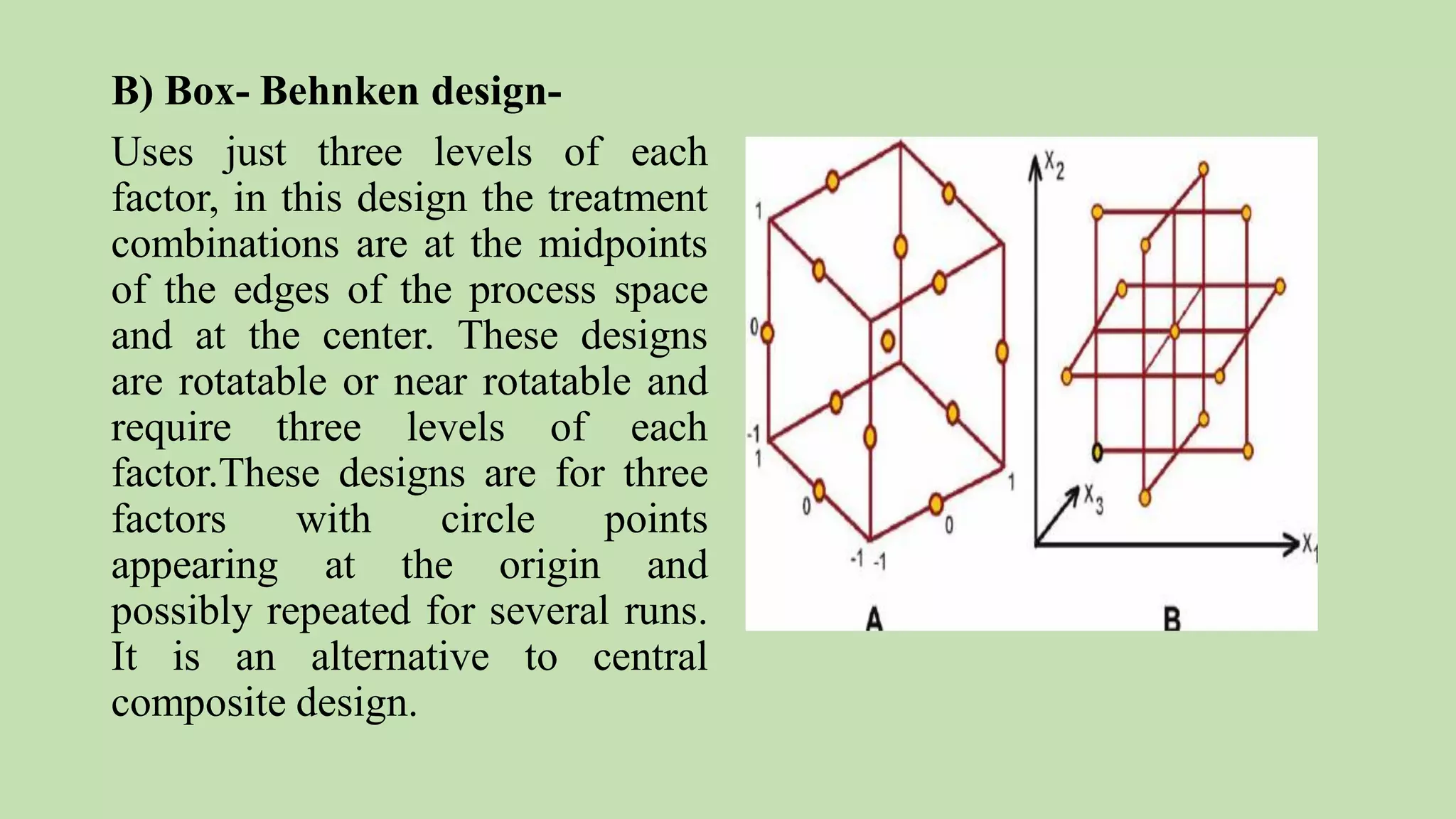
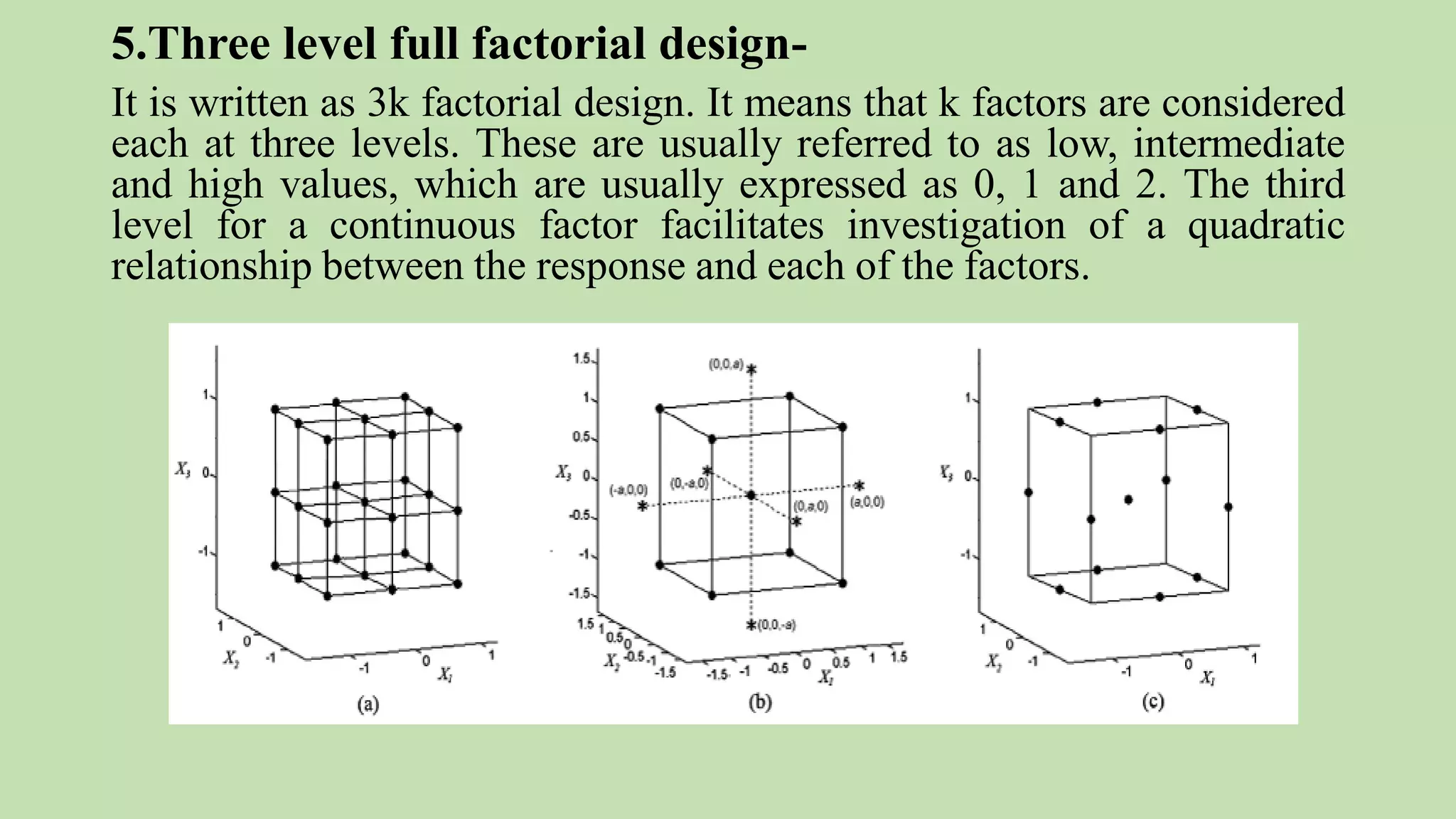
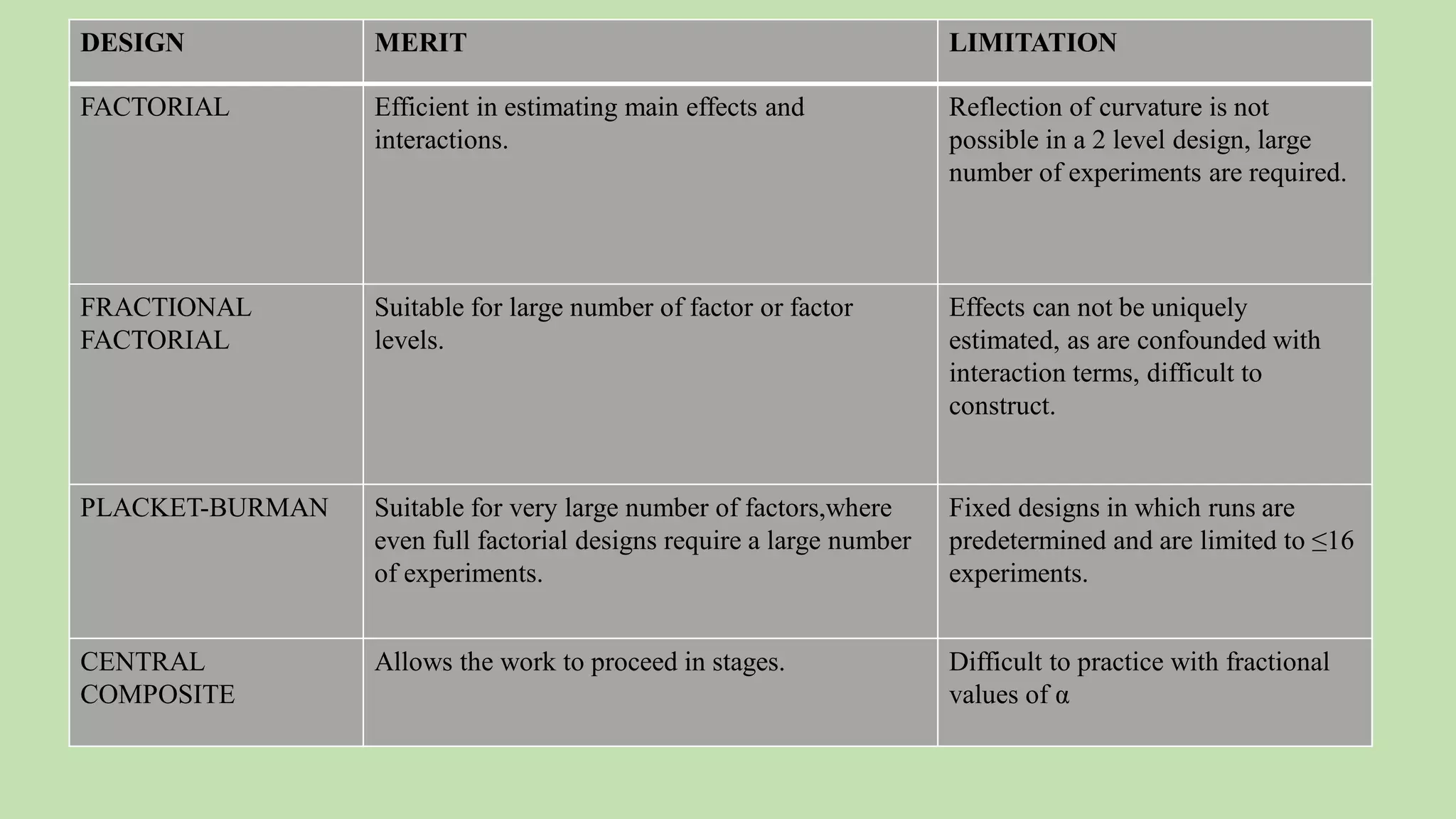
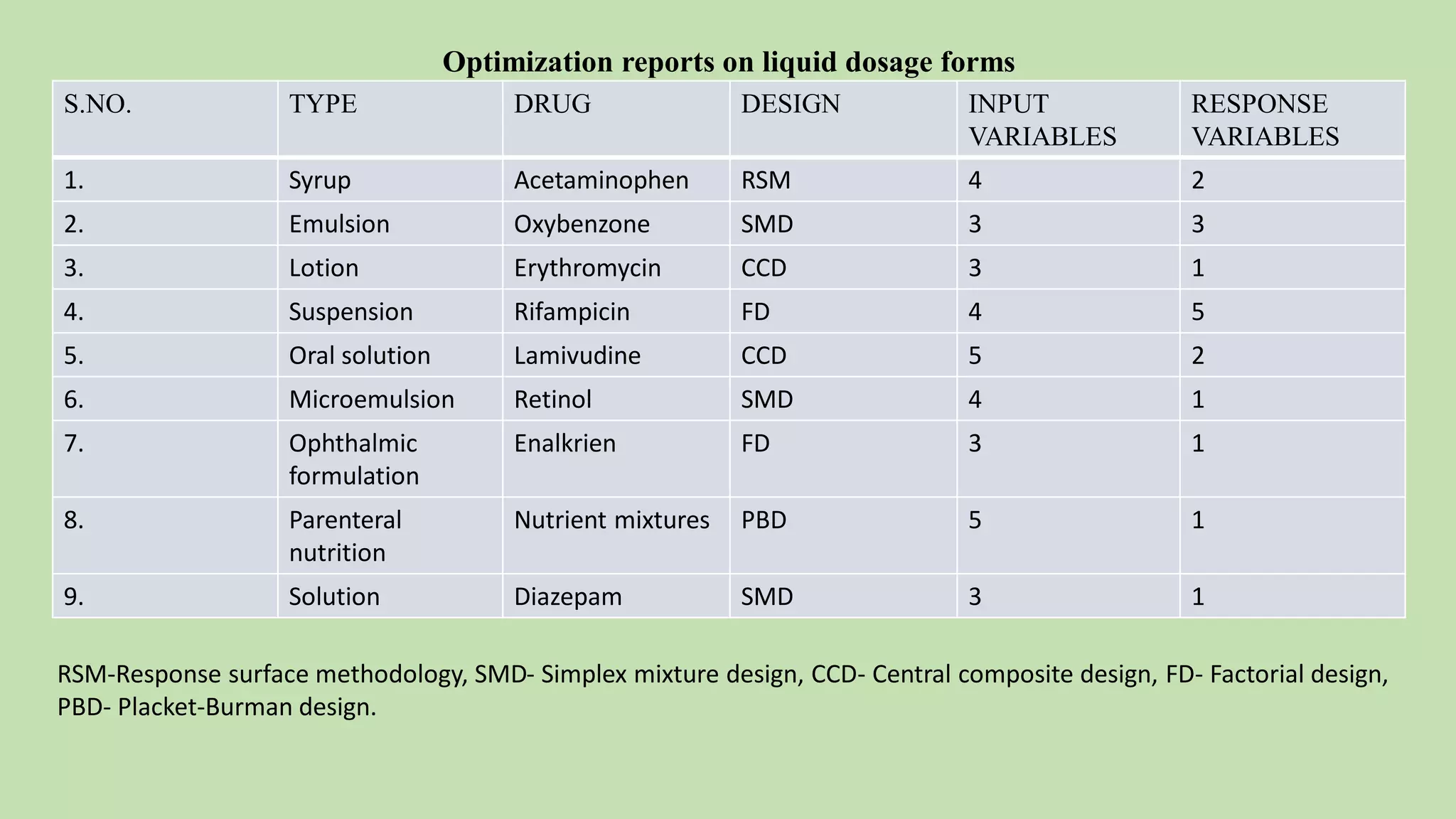
Computer-aided formulation development involves using computers to optimize drug products and processes. It involves several variables that are optimized using experimental designs like factorial designs. Computers are used at every step of optimization including design selection, data analysis, and result interpretation. Common software used include Design Expert, MINITAB, and JMP. Reports have optimized various liquid dosage forms like emulsions and suspensions. Computers also play a role in intellectual property protection of innovative research. Ethical issues involving privacy and codes of conduct must also be considered with computational research in pharmaceuticals. Computers can analyze market data to inform business decisions.