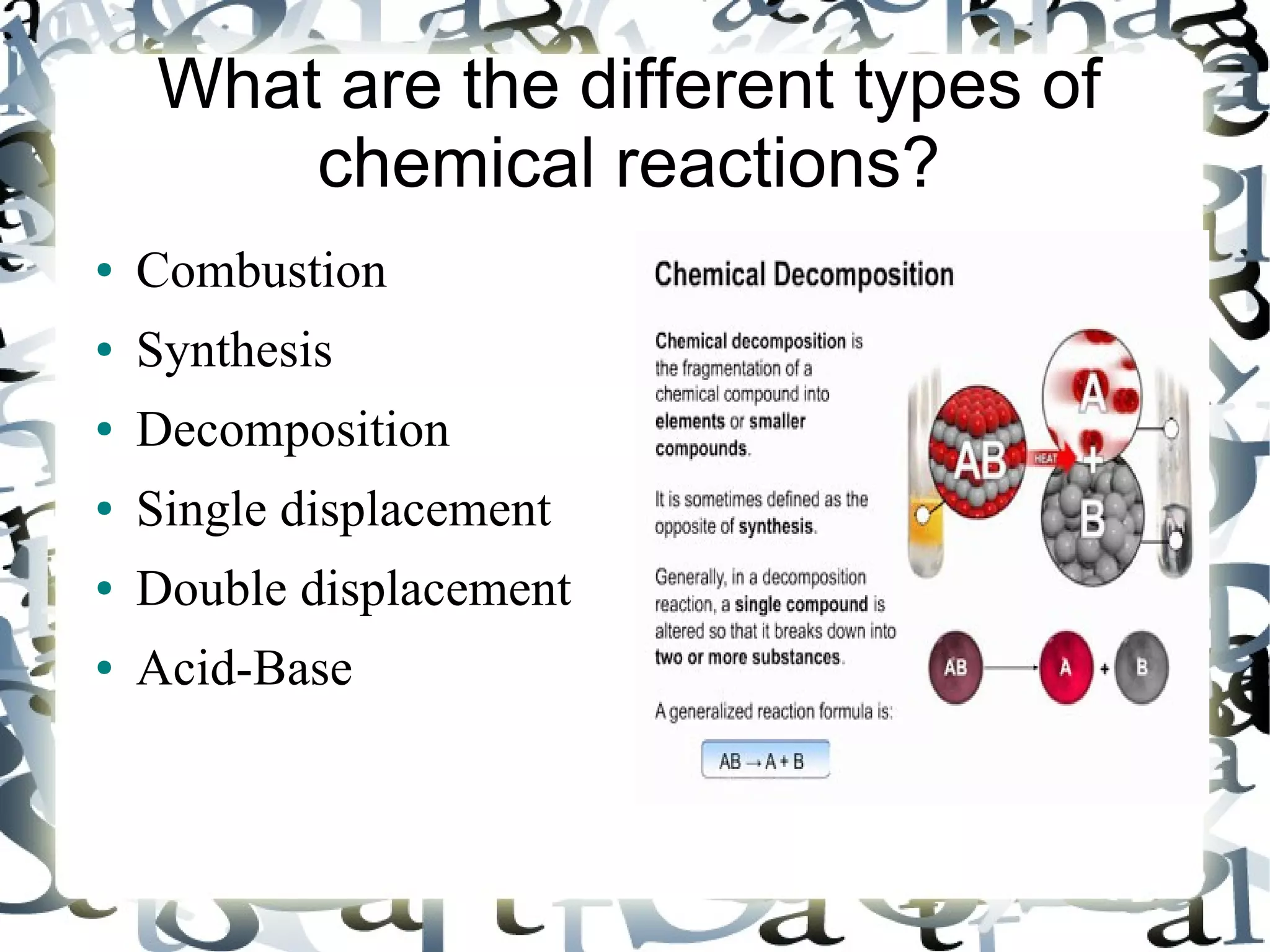
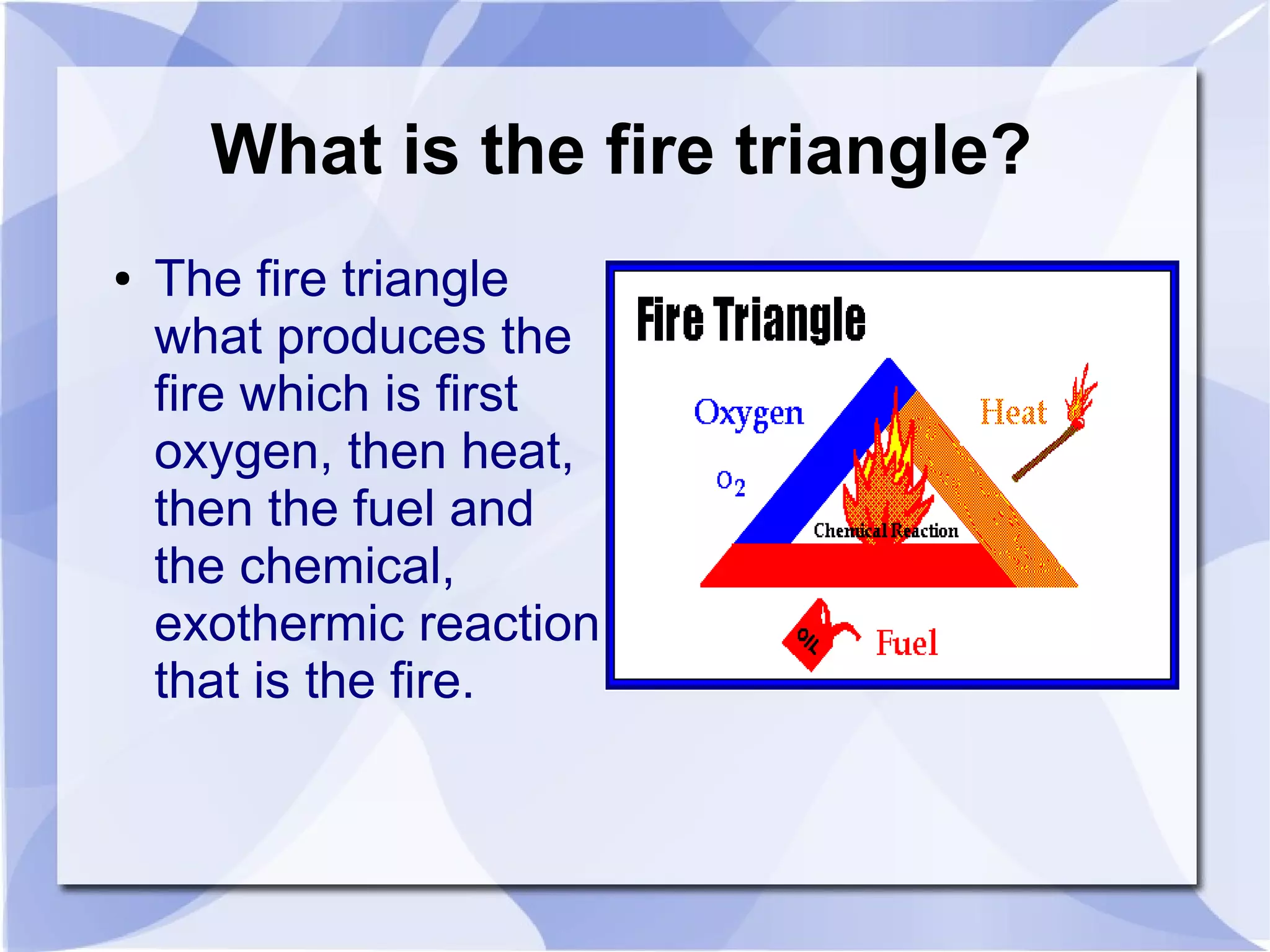
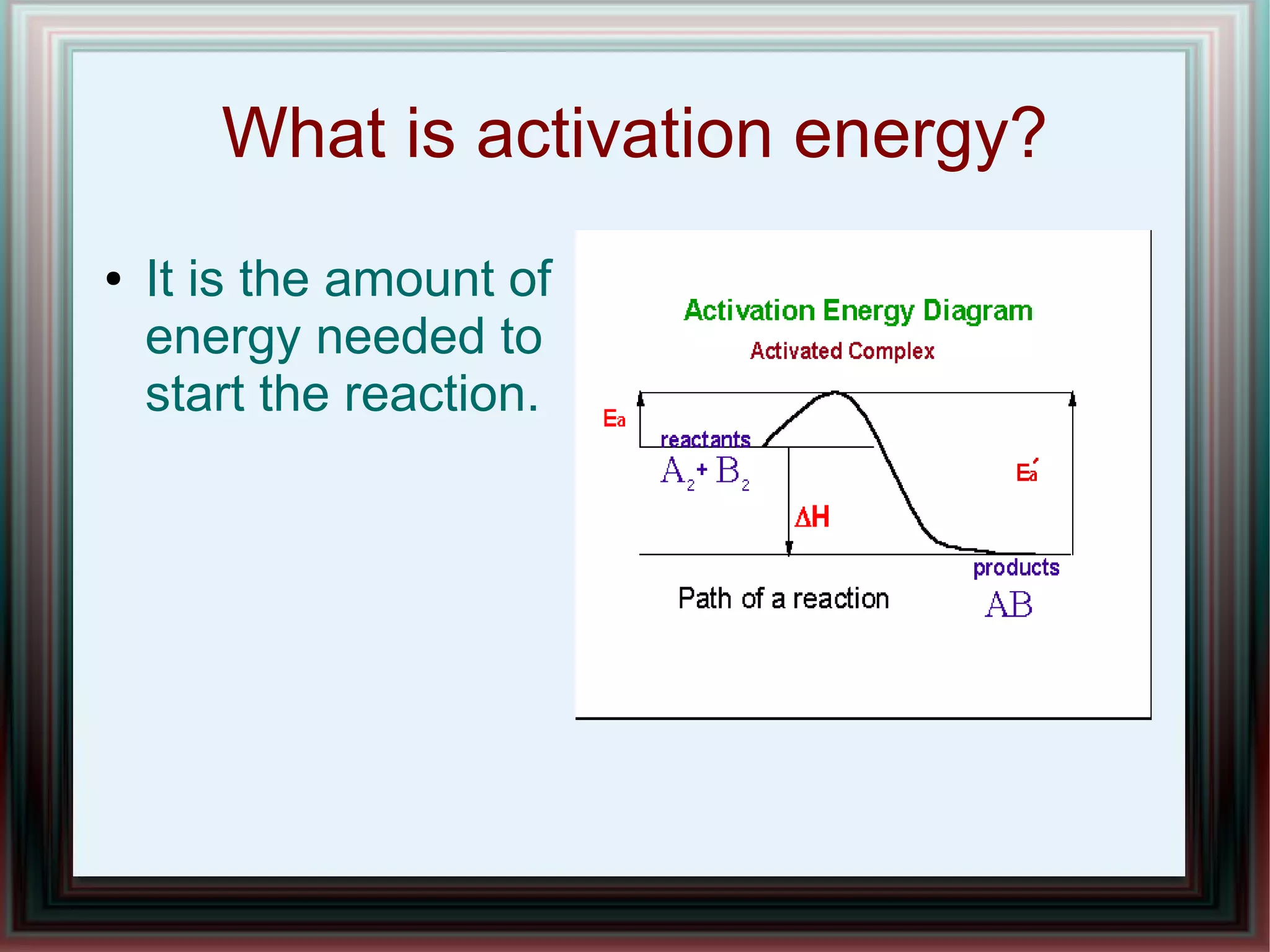
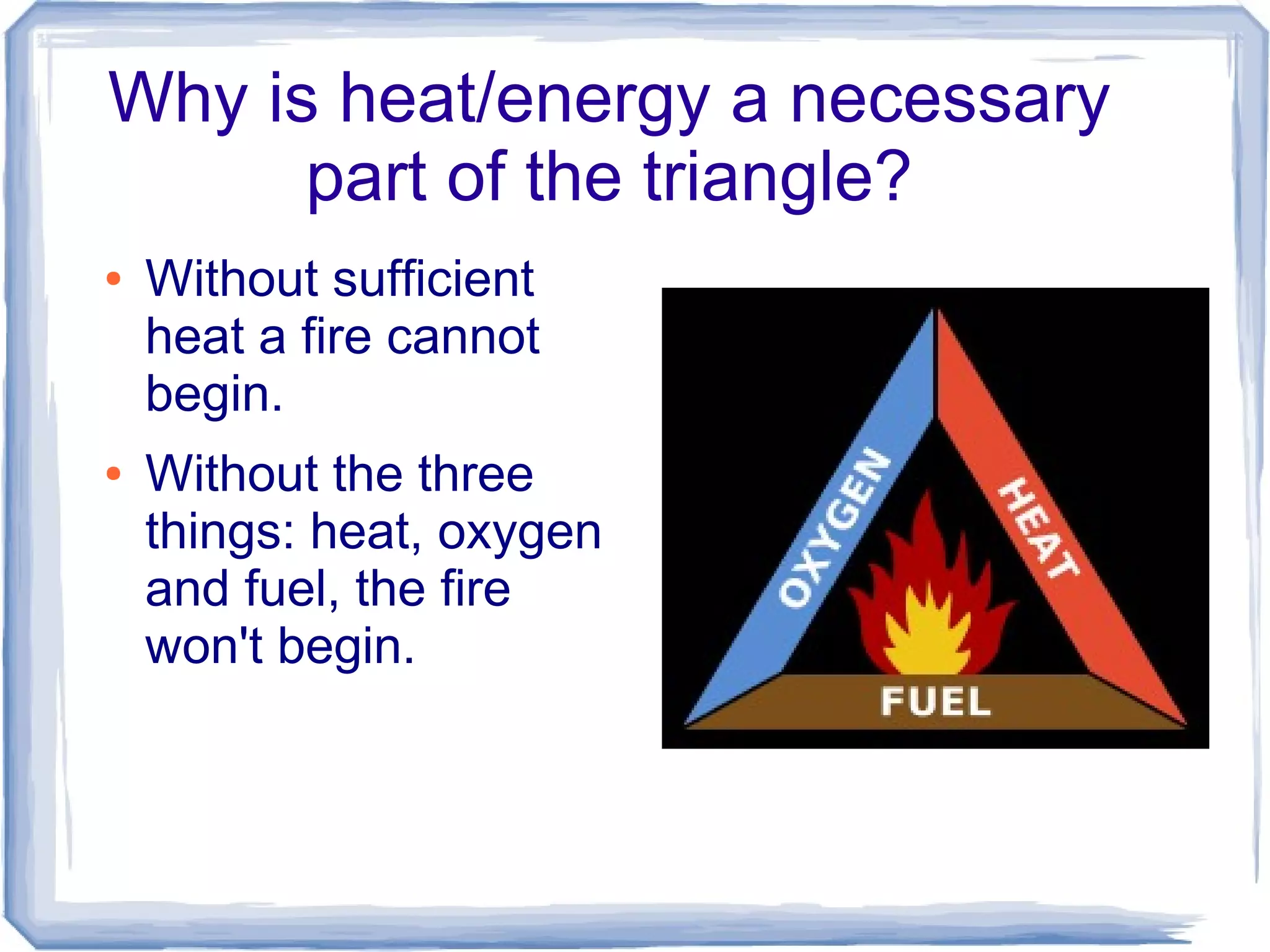
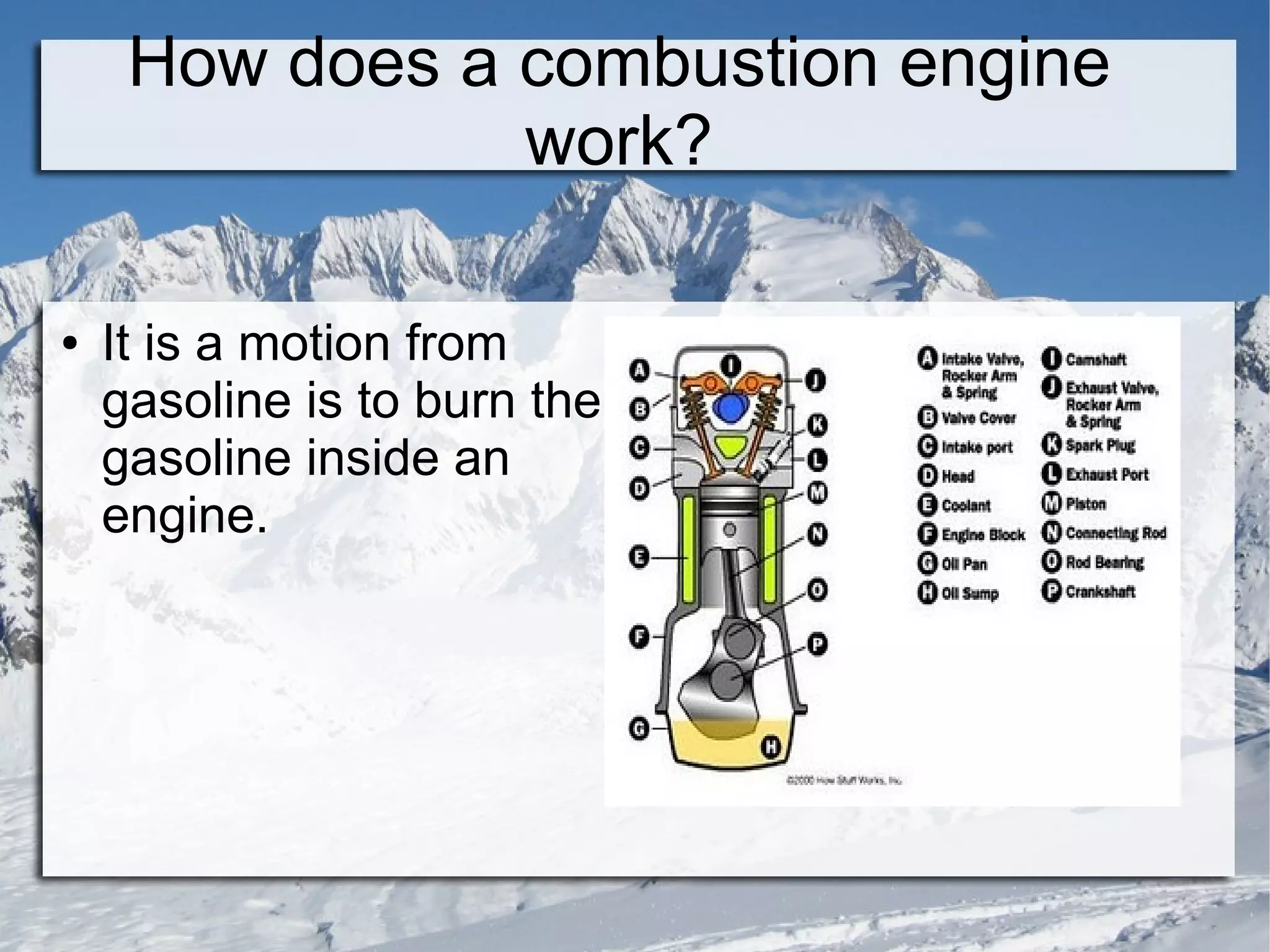
The document discusses combustion reactions and provides examples. A combustion reaction occurs when a fuel and oxidant react, producing heat or heat and light. The fire triangle represents the three components needed for fire: oxygen, heat, and fuel. Activation energy is the minimum amount of energy needed to start a combustion reaction. A combustion engine uses the combustion of fuel to create motion, such as powering a vehicle. NASCAR engines differ from street car engines in that they have more combustion due to operating at higher speeds. Nitrous oxide can improve engine performance by providing more oxygen during combustion, allowing the engine to produce more power. The document also discusses penalties imposed by NASCAR for cheating that provided aerodynamic advantages.