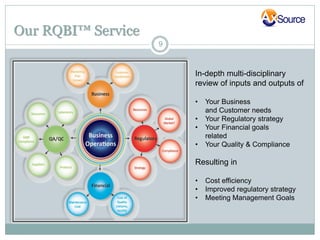
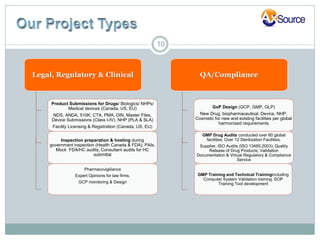
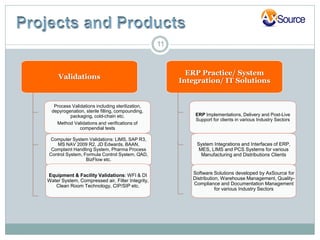
AxSource is a healthcare and information technology solutions provider specializing in consulting services for various industries including pharmaceutical, biologics, medical devices, and food. They provide regulatory, quality, engineering and IT consulting services to help clients with activities like new product launches and emerging market operations. AxSource consists of highly qualified consultants with decades of experience in North America, Europe, Mexico, and Asia.