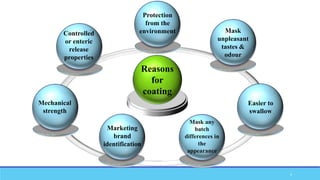
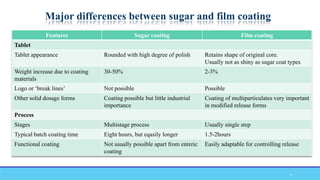
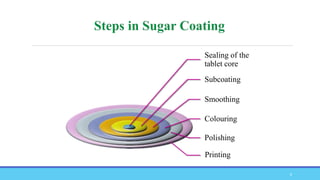
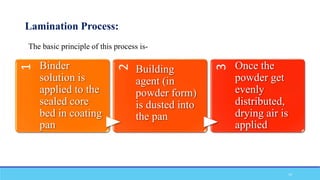
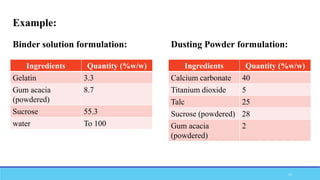
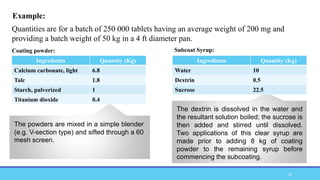
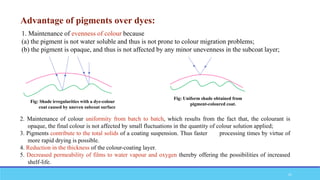
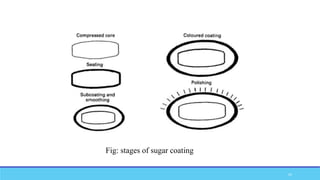
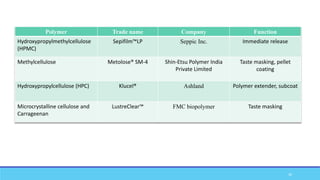
The document discusses tablet coating processes, emphasizing the importance of both sugar and film coatings for enhancing tablet properties like appearance, taste masking, and controlled release. It outlines the stages and materials involved in sugar and film coating, including the benefits and challenges of each method, as well as the characteristics of various polymers used in film coatings. Overall, it highlights the evolution of coating techniques and their significance in pharmaceutical formulations.







![[1] Shellac
This is a purified resinous secretion of the insect Laccifer lacca, indigenous to
India.
Solubility: Shellac is insoluble in water but shows solubility in aqueous alkalis; it
is moderately soluble in warm ethanol.
Problems:
material of natural origin and consequently suffers from occasional supply problems
quality variation
stability problems associated with increased disintegration and dissolution times on
storage.
Polymer Trade name Function
Shellac EmCoat 120 N
MarCoat 125
Enteric coat
11](https://image.slidesharecdn.com/finalcoatingassignment-220708200703-6d2938a6/85/Coating-of-Pharmaceutical-Tablet-11-320.jpg)
![[2] Zein
It is a protein contained in the endosperm tissue of Zea mais occurs as by product
of corn processing.
Solubility: soluble in alcohol.
Polymer Trade name Function
Zein G200 Enteric coat
12](https://image.slidesharecdn.com/finalcoatingassignment-220708200703-6d2938a6/85/Coating-of-Pharmaceutical-Tablet-12-320.jpg)












![[1] Acrylic Polymer/ methacrylate aminoester copolymer
Insoluble in water
Soluble in Acidic medium having pH <4
Solubility in neutral and alkaline medium is achieved by swelling and
increased permeability to aqueous medium.
Complete disintegration and dissolution of film can be assured by
incorporation of soluble polymers such as cellulose ethers, starch.
Polymer Trade name Company Function
Copolymers of acrylate and
methacrylates with quarternary
ammonium group in
combination with sodium
Carboxymethylcellulose
Eudragit RD 100 Evonik Rapidly disintegrating
34](https://image.slidesharecdn.com/finalcoatingassignment-220708200703-6d2938a6/85/Coating-of-Pharmaceutical-Tablet-34-320.jpg)
![[2] Cellulose derivatives
Majority of these polymers used in film coating are ethers of cellulose.
Polymer chain length together with size and extent of branching will
determine the viscosity of polymer solution.
Polymer Substituent Solubility Other properties
HPMC (Hydroxy Propyl
Methyl Cellulose)
-CH3-CH2-CH(OH)-CH3 Soluble in both aqueous and
organic solvents
• Non-tackiness
• Flexible strong film
Hydroxy ethyl cellulose -CH(OH)-CH3 Soluble in water,
Insoluble in organic solvents
Hydroxy propyl
cellulose
—CH2 —CH(OH)—CH3 Soluble in aqueous and
alcoholic media.
• Tackiness
• Weak film
Methyl cellulose -CH3 It has LCST 40-50˚C.
Readily soluble in water below
it’s LCST.
Rarely used in film coating due to
lack of commercial availability of
low viscosity material meeting
appropriate compebdial
requirements
35](https://image.slidesharecdn.com/finalcoatingassignment-220708200703-6d2938a6/85/Coating-of-Pharmaceutical-Tablet-35-320.jpg)

![[1] Methacrylate ether copolymer
Completely esterified with no free carboxylic acid group.
Neutral in nature and insoluble over entire physiological pH.
Become swell and permeable to water and dissolve substances thus used in
modified release formulation, e.g. Eudragit
Extended release coating polymer
Polymer Trade name Company Function
Copolymers of
acrylate and
methacrylates with
quarternary
ammonium group.
Insoluble, High permeability:
Eudragit® RL PO, Eudragit® RL,
Eudragit® RL 30D
Insoluble,Low Permeability:
Eudragit® RSPO, Eudragit® RS
30D
Evonik Sustained release
38](https://image.slidesharecdn.com/finalcoatingassignment-220708200703-6d2938a6/85/Coating-of-Pharmaceutical-Tablet-38-320.jpg)
![[2] Ethyl Cellulose
High substitution of cellulose make it insoluble in water.
The polymer is not usually used on its own but normally in combination with
secondary polymers such as HPMC etc.
Ideal polymer for modified release coating due to following properties:
Odourless
Tasteless
High degree of stability (both under physiological conditions and normal
storage condition)
Good solubility in common solvents used in coating.
Polymer Trade name Company Function
Ethyl cellulose Aquacoat® ECD FMC Sustained release
Surelease® Colorcon
39](https://image.slidesharecdn.com/finalcoatingassignment-220708200703-6d2938a6/85/Coating-of-Pharmaceutical-Tablet-39-320.jpg)
![Enteric coating polymer
[1] Methacrylic acid copolymer
These polymer possess free carboxylic acid group.
They form salt with alkalis and having solubility at pH in excess of 5.5
Depending on the degree of substitution with carboxylic group they have
different pH-dissolution profile.
Specific name Monomers Trade name Marketed form
Poly(methylacrylate,
ethylacrylate)
copolymer of MA and EA in a
molar ratio of 1:1
Eudragit L30D 30% aqueous dispersion
Eudragit L100-55 Powder
Poly(methacrylic acid,
methylmethacrylate)
copolymer of MA and MMA
in a molar ratio of 1:1
Eudragit L12.5 12.5% solution in isopropanol
Eudragit L100 Powder
Poly(methacrylic acid,
methylmethacrylate)
copolymer of MA and MMA
in a molar ratio of 1:2
Eudragit S12.5 12.5% solution in isopropanol
Eudragit S100 Powder
40](https://image.slidesharecdn.com/finalcoatingassignment-220708200703-6d2938a6/85/Coating-of-Pharmaceutical-Tablet-40-320.jpg)
![[2] Cellulose esters
Polymer Substituent Solubility Other properties Trade name
Cellulose acetate
phthalate (CAP)
-CO-CH3, CO-C6H4-COOH Insoluble in water &
alcohol
• Oldest and widely
used enteric coat.
• Prone to hydrolysis
Aquacoat CPD®,
C–A–P NF
Eastman
Cellulose acetate
trimellitate (CAT)
-CO-CH3,-CO-C6H3-
(COOH)2
Start to dissolve at low
pH 5.5 thus efficient
dissolution in upper
small intestine
Chemically resembles
CAP but have
additional -COOH
group
Hydroxy propyl
methyl cellulose
phthalate (HPMCP)
-CH3,-CH2CH(OH)CH3,-
CO-C6H4-COOH
Insoluble in water but
soluble in aqueous
alkalis & acetone/water
95:5 mixture
More flexible polymer InstacoatTM EN-
HPMCP
41](https://image.slidesharecdn.com/finalcoatingassignment-220708200703-6d2938a6/85/Coating-of-Pharmaceutical-Tablet-41-320.jpg)

![Solid dosage form may be incorporated with several special materials such as:
[1] Flavours and sweeteners are added to mask unpleasant odours or to develop the taste. e.g., fruit
spirits (organic solvent), aspartame, water soluble pineapple flavour.
[2] Surfactants are ancillary to stabilize immiscible or insoluble ingredients in the coating. They
facilitate substrate wettability and promote coalescence of polymeric material over the substrate’s
surface e.g., Spans, Tweens etc.
[3] Antioxidants are incorporated to stabilize a dye system to oxidation and colour change e.g.,
oximes, phenols etc.
[4] Antimicrobials are added to inhibit microbial growth in the coating composition. Various
cellulosic materials are mainly prone to microbial growth and they can not be stored in solution
form e.g., Carbamates, alkylisothiazloinone, benzothiazoles etc.
MiscellaneousCoating Solution Components
53](https://image.slidesharecdn.com/finalcoatingassignment-220708200703-6d2938a6/85/Coating-of-Pharmaceutical-Tablet-53-320.jpg)
