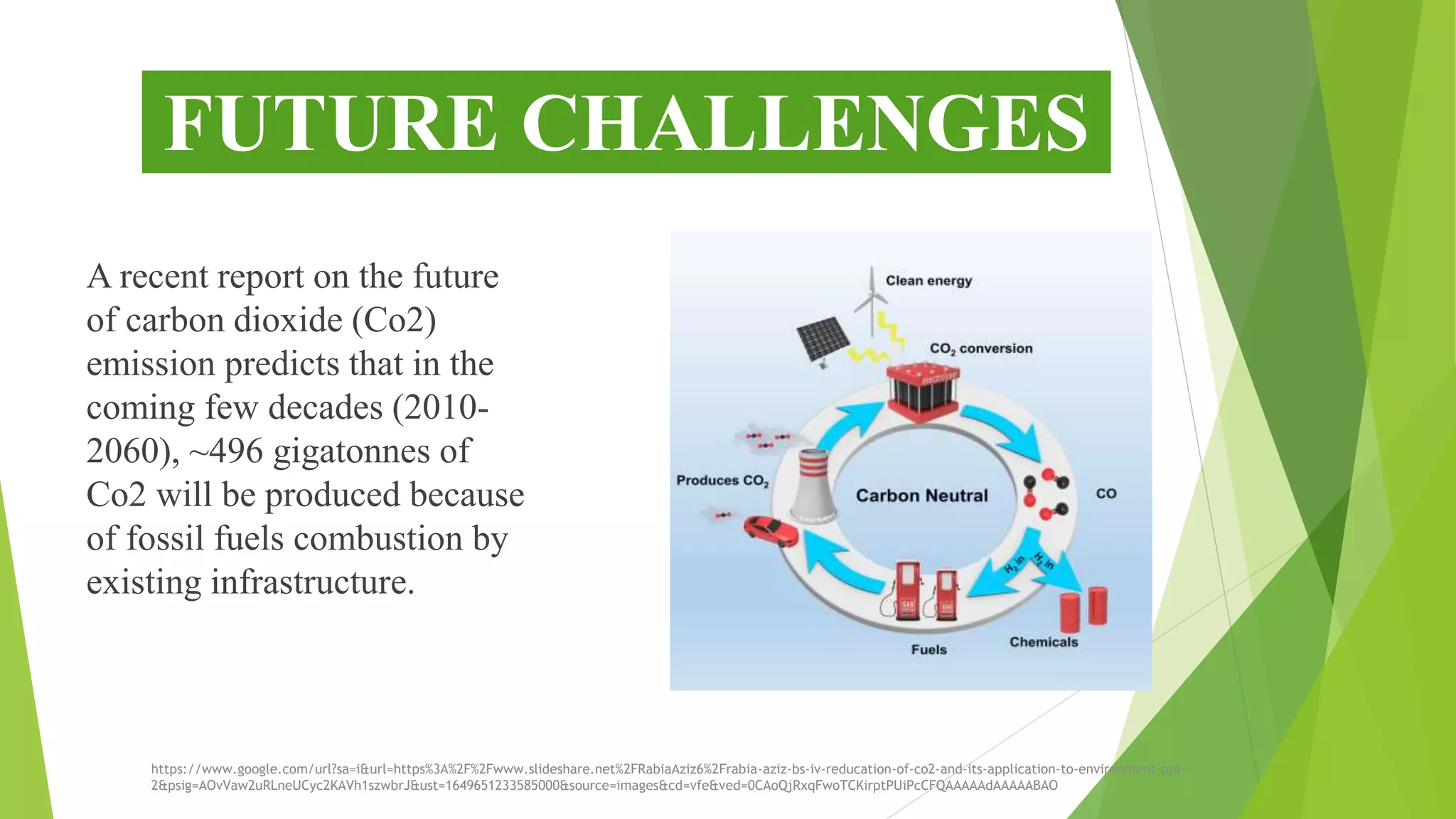
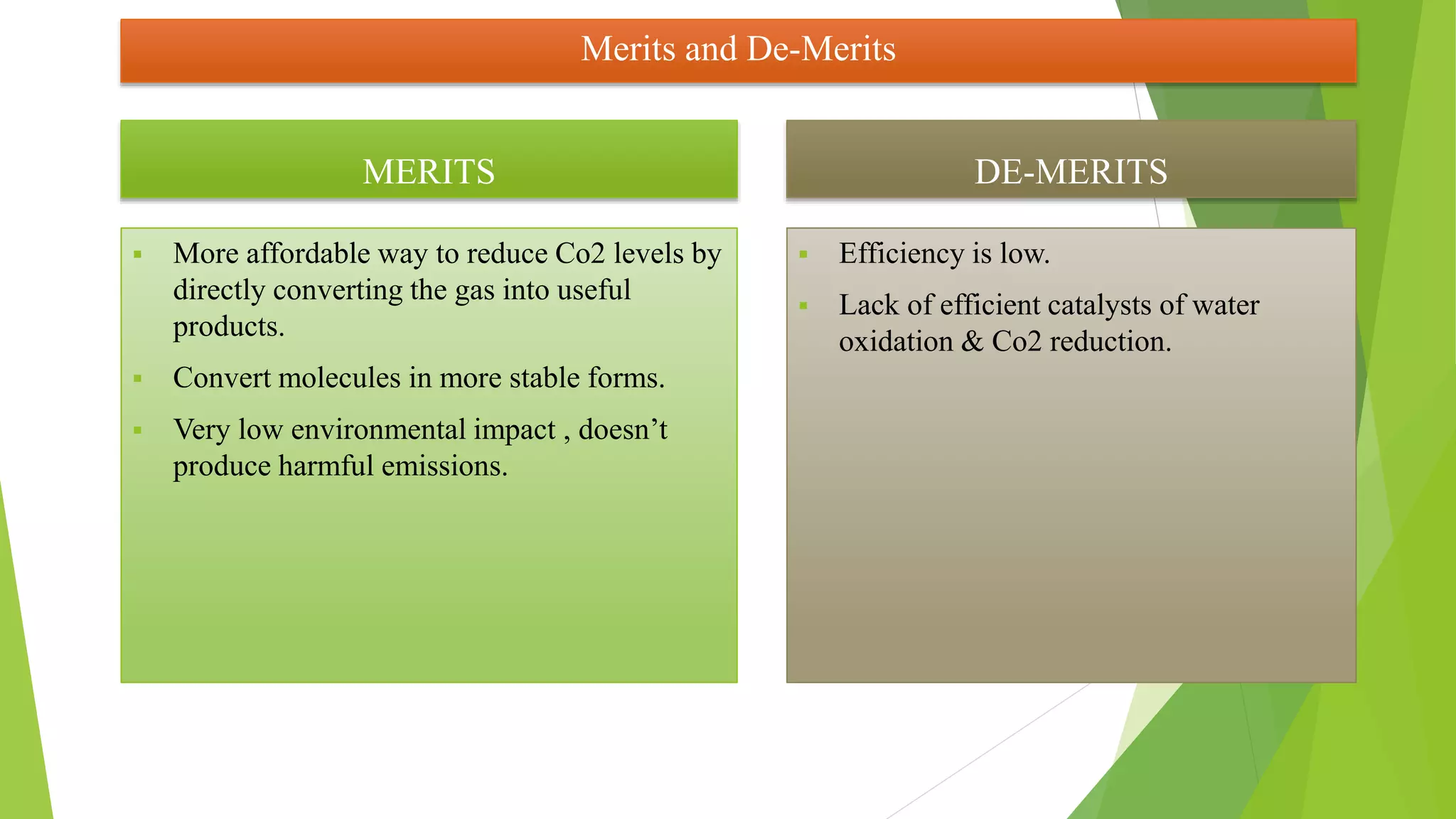
The document discusses methods for reducing carbon dioxide (CO2) emissions, highlighting both artificial and natural solutions. It examines various reduction techniques, including electrochemical and photochemical methods, and outlines the role of catalysts in converting CO2 into valuable chemicals and fuels. Additionally, it addresses the challenges and benefits associated with CO2 reduction, emphasizing the need for efficient catalysts and the low environmental impact of these processes.